StemXVivo Cardiomyocyte Differentiation Kit
R&D Systems, part of Bio-Techne | Catalog # SC032B

Key Product Details
Assay Procedure
Refer to the product datasheet for complete product details.
Briefly, human pluripotent stem cells are differentiated into the cardiomyocyte lineage using the following differentiation procedure:
- Plate cells on coated plates
- Replace MEF Conditioned Media with Cardiomyocyte Differentiation Base Media I containing RGF BME
- Replace the media with Day 1 Differentiation Media
- Replace the media with Day 5 Differentiation Media
- Replace the media with Cardiomyocyte Differentiation Base Media II
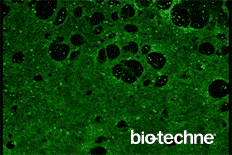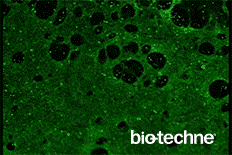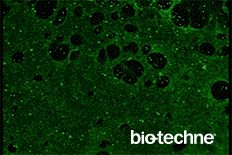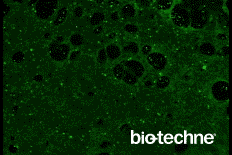
- Evaluate differentiation status using the included Troponin T antibody
- Cells are ready for downstream applications
Reagents supplied in the StemXVivo® Cardiomyocyte Differentiation Kit (Catalog # SC032).
- Stem Cell Qualified RGF BME, Pathclear®
- Cardiomyocyte Differentiation Base Media Supplement I
- Cardiomyocyte Differentiation Base Media Supplement II
- Cardiomyocyte Differentiation Cocktail I
- Cardiomyocyte Differentiation Cocktail IIA
- Cardiomyocyte Differentiation Cocktail IIB
- Cardiomyocyte Differentiation Cocktail III
- Anti-Human Cardiac Troponin T Antibody
Reagents
- RPMI 1640
- BSA, very low endotoxin
- D-MEM/F-12 (1X)
- GlutaMAX™ (Invitrogen, Catalog # 35050-079 or equivalent)
- Penicillin-Streptomycin (optional)
- Phosphate Buffered Saline (PBS)
- 95% Ethanol
- 4% Paraformaldehyde
- 1% BSA in PBS
- 0.3% Triton™ X-100, 1% BSA, 10% normal donkey serum in PBS
- Mounting medium (R&D Systems, Catalog # CTS011)
- Secondary developing reagent (R&D Systems, Catalog # NL001)
- Deionized or distilled water
Materials
- Human pluripotent stem cells
- 24-well culture plates (or other, as needed)
- 60 mm culture plates
- 12 mm coverslips (optional)
- 15 mL and 50 mL centrifuge tubes
- 0.2 μm syringe filter
- 10 mL syringe
- Pipettes and pipette tips
- Serological pipettes
- Glass slides
- Fine pointed curved forceps
- FACS tubes
- Flow Cytometry Fixation/Permeabilization Buffer I (R&D Systems, Catalog # FC007) supplemented with 0.1% Triton X-100
- Flow Cytometry Permeabilization/Wash Buffer I (R&D Systems, Catalog # FC005)
Equipment
- 37 °C and 5% CO2 incubator
- 37 °C water bath
- Centrifuge
- Inverted microscope
- Fluorescence microscope
Precaution: The acute and chronic effects of overexposure to reagents of this kit are unknown. Safe laboratory procedures should be followed and protective clothing should be worn when handling kit reagents.
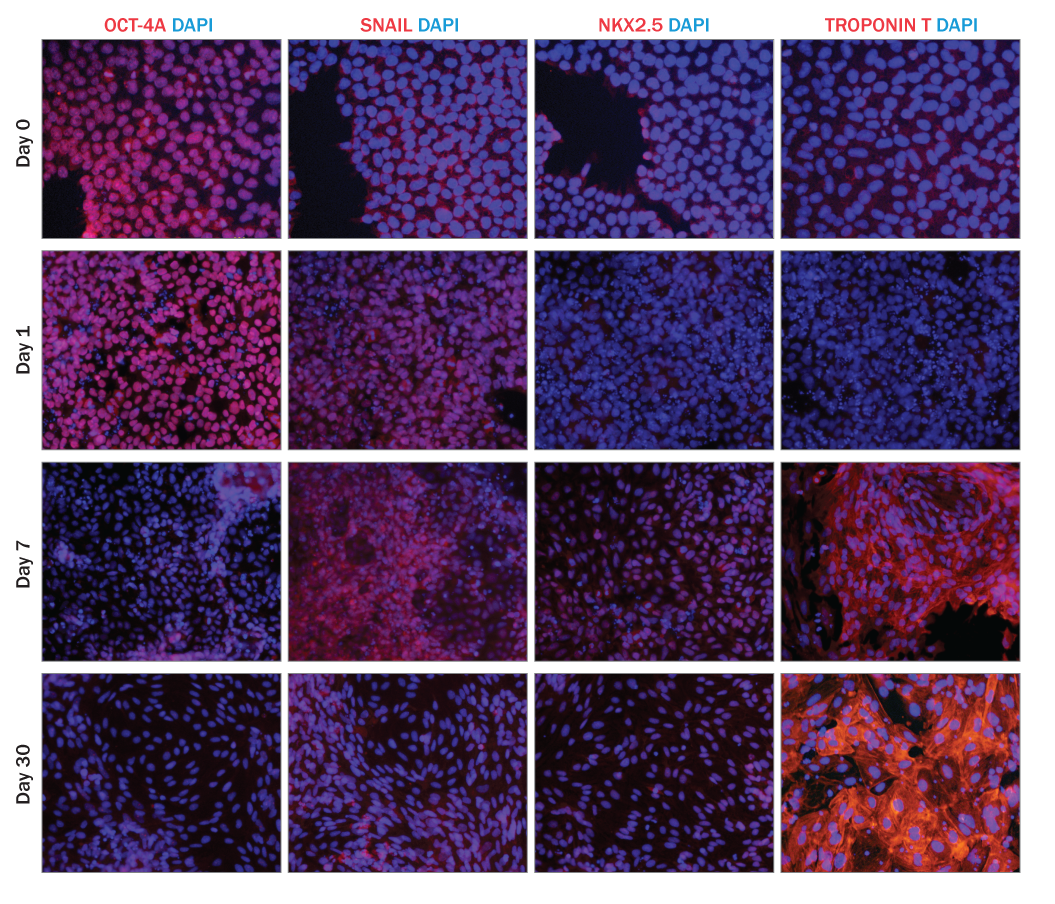
This protocol has been tested on human pluripotent stem cells cultured in either MEF Conditioned Media (R&D Systems, Catalog # AR005) or equivalent. The quality and differentiation potential of human pluripotent stem cells at the onset of the differentiation protocol are of paramount importance to the efficiency of differentiation. Human pluripotent stem cells must be > 95% positive for OCT-3/4.
Coat wells with Stem Cell Qualified PathClear® RGF BME (RGF BME).
Incubate at room temperature for 1-2 hours.

Plate human pluripotent stem cells onto the coated plates at 3-4 x 104 cells/cm2 in Pluripotent Stem Cell Maintenance Media.
Culture cells to 80-90% confluency.

Day (-1) of Differentiation
Replace the stem cell culture media with ice cold Pluripotent Stem Cell Maintenance Media containing RGF BME diluted 1:60.
Incubate at 37 °C and 5% CO2 for 18-24 hours.

Day 0 of Differentiation
Replace the media with ice cold Day 0 Cardiomyocyte Differentiation Media containing RGF BME diluted 1:60.
Incubate at 37 °C and 5% CO2 for 24 hours.

Day 1 of Differentiation
Replace the media with Day 1 Cardiomyocyte Differentiation Media
Incubate at 37 °C and 5% CO2 for 4 DAYS without media exchange.

Day 5 of Differentiation
Replace the media with Day 5 Cardiomyocyte Differentiation Media.
Incubate at 37 °C and 5% CO2 for 2 DAYS without media exchange.

Day 7 of Differentiation and Beyond
Replace the media with Cardiomyocyte Differentiation Base Media I.
Incubate at 37 °C and 5% CO2. Replace media every 1-2 days as needed.

Day 12 of Differentiation and Beyond
Replace the media with Cardiomyocyte Differentiation Base Media II.
Incubate at 37 °C and 5% CO2. Replace media every 1-2 days as needed.

Customer Reviews for StemXVivo Cardiomyocyte Differentiation Kit
There are currently no reviews for this product. Be the first to review StemXVivo Cardiomyocyte Differentiation Kit and earn rewards!
Have you used StemXVivo Cardiomyocyte Differentiation Kit?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review