Meet the Maurice Family
Capillary electrophoresis instruments for accelerating biologics development.
Analyze monoclonal antibodies (mAbs), viral particles, proteins antibody drug conjugates (ADCs), and more.
- Analyze protein charge with imaged capillary isoelectric focusing (icIEF)
- Analyze protein size and impurities with capillary electrophoresis-SDS (CE-SDS)
- Collect charge isoform fractions with icIEF-based fractionation for further characterization using the MauriceFlex
How Maurice Technology Works
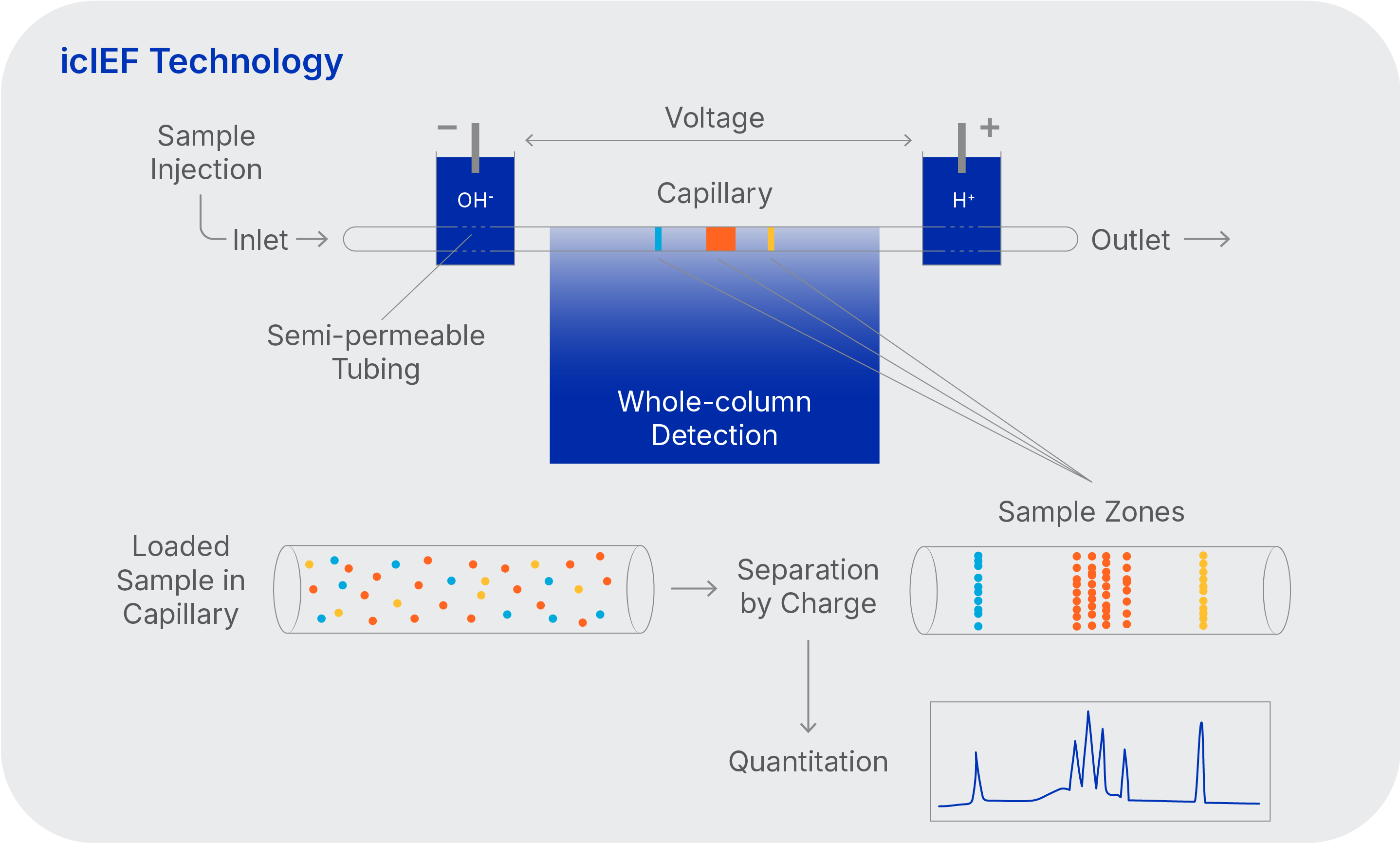
icIEF technology for analyzing charge heterogeneity of therapeutic proteins
- Whole-column imaging
- No extra mobilization step after focusing
- High resolution
- Quantitative data
- Greener method than HPLC
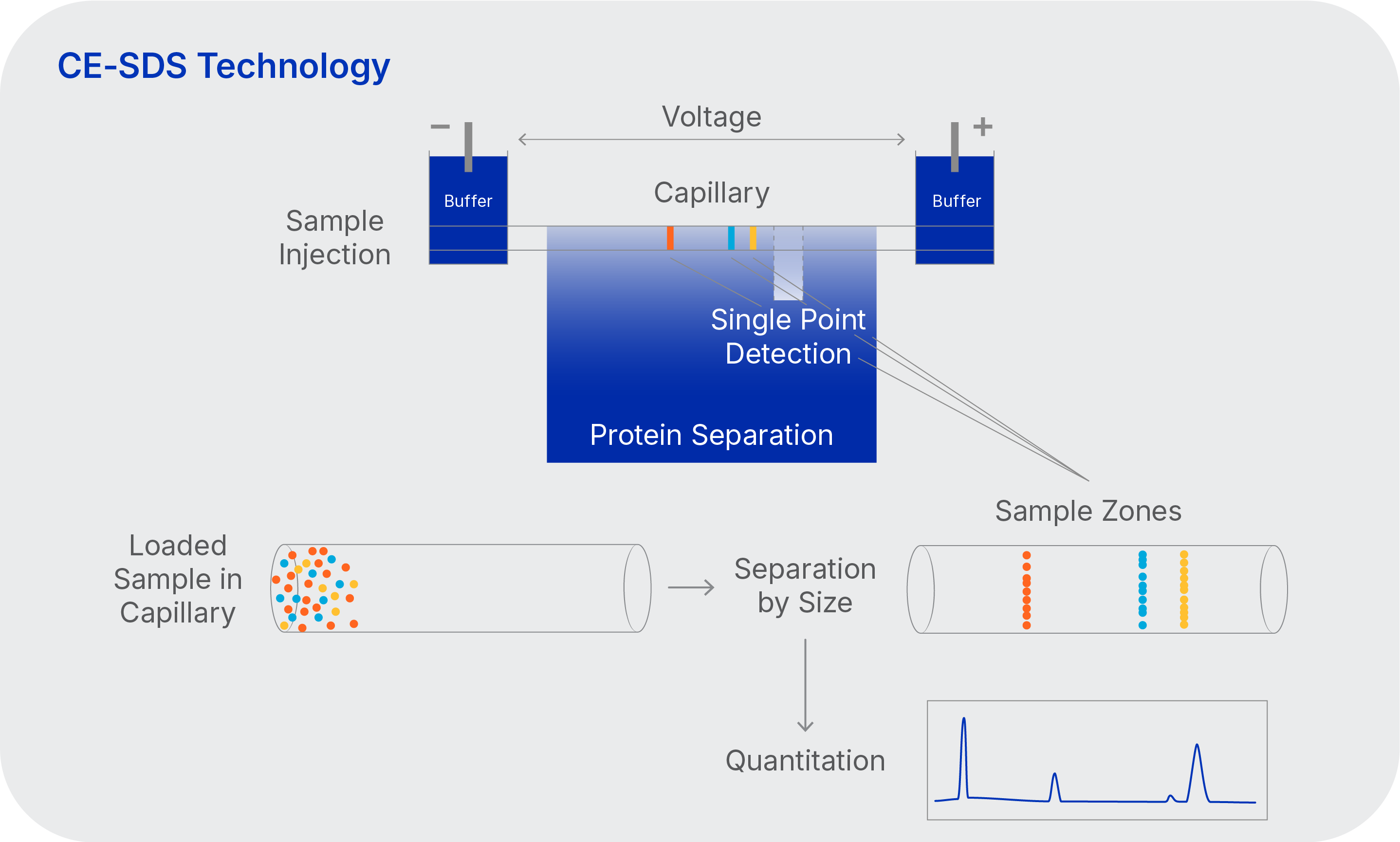
CE-SDS technology for analyzing the size and purity of therapeutic proteins
- Simplified sample prep and automated injection
- No acrylamide
- Quantitative data
- Greener method than SDS-PAGE
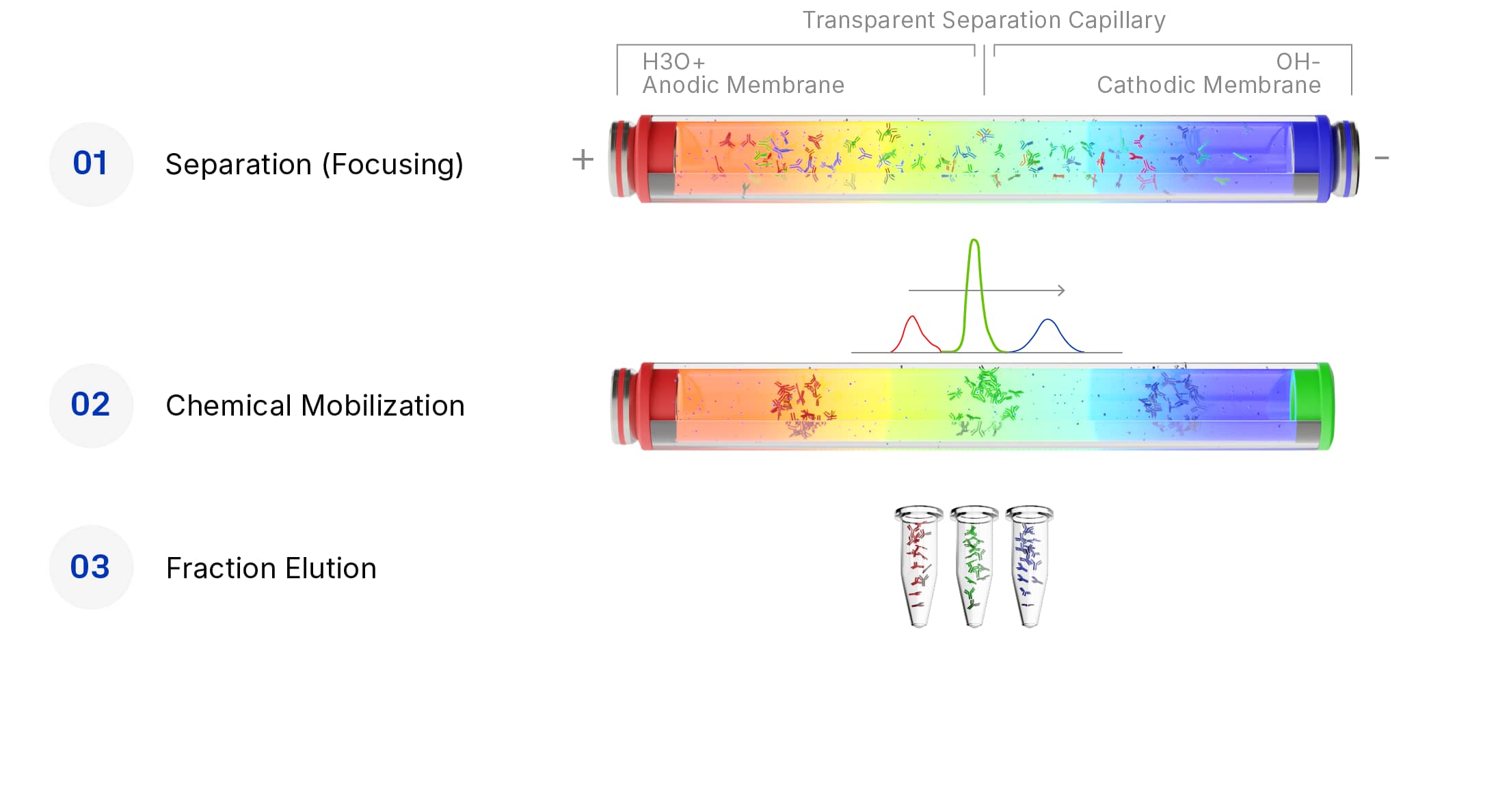
How Biomolecular and Protein Fractionation Works on MauriceFlex
Get deeper characterization of charge variants with fractionation
- An applied electric field creates a pH gradient across the capillary inside the MauriceFlex cartridge
- Proteins separate by migrating to different pH values on the gradient, based on their individual isoelectric point (pI)
- Introduction of ammonium acetate chargers the pH gradient, mobilizing these separated proteins and results in elution (fractionation)
Explore the Maurice Capillary Electrophoresis System
From sample prep to data analysis, take a virtual tour and explore iCIEF and CE-SDS workflows on Maurice.
Capillary Electrophoresis Applications with Maurice
Charge, size, and charge variant analysis and fraction collection for mAbs, ADCs, AAVs, and more.
| System | MauriceFlex | Maurice | Maurice C. | Maurice S. |
|---|---|---|---|---|
| icIEF Charge Application | Yes | Yes | Yes | No |
| CE-SDS Size Application | Yes | Yes | No | Yes |
| icIEF Fractionation | Yes | No | No | No |
| Onboard Mixing for Sample Prep | N/A | Yes | Yes | No |
| Absorbance Detection | Yes | Yes | Yes | Yes |
| Native Fluorescence Detection | Yes | Yes | Yes | No |
Capillary Electrophoresis with Maurice Resources
Consumables
- Get everything you need to run Maurice instruments
Software
- Control your systems and maintain data integrity with a range of industry-validated software
Citations
- Explore how the Maurice systems are empowering labs worldwide in different fields
Maurice Family Brochure
- Learn about the technology, product offerings, and relevant data
All Maurice Literature
- Browse through our library of whitepapers, application notes, and posters that cover different biotherapeutic molecules and research areas

Learn More About Image Capillary Electrophoresis (ICE) Innovation
eNewsletter
eNewsletter
Stay up to date and sign up now to subscribe to CE Chronicles eNewsletter. Read featured articles on biotherapeutic characterization, get the latest in industry news, learn about new products, discover what your peers are up to, and more.
VIrtual Event
VIrtual Event
As part of our Celebration of 25 years of iCE technology, catch up on the full capabilities of the Maurice platforms. Watch the webinar: Latest Advances in Biotherapeutic Characterization.
Hear from Maurice Users
Hear from Sara Carillo, NIBRT talk about her lab’s experience with the MauriceFlex system, and how it accelerated the characterization of charge variants.
Hear from Siddharth Sapa, Teva Pharmaceuticals, describe the benefits of using the Maurice system and the icIEF 400 cartridge, including high-throughput and ease-of-use.