Recombinant Human Thrombopoietin/TPO Protein, Animal-Free Best Seller
R&D Systems, part of Bio-Techne | Catalog # BT-TPO-AFL

Key Product Details
Product Specifications
Source
E. coli-derived human Thrombopoietin/Tpo protein
Ser22-Leu195
Produced using non-animal reagents in an animal-free laboratory.
Ser22-Leu195
Produced using non-animal reagents in an animal-free laboratory.
Purity
>95%, by SDS-PAGE with quantitative densitometry by Coomassie® Blue Staining.
Endotoxin Level
<0.10 EU per 1 μg of the protein by the LAL method.
N-terminal Sequence Analysis
Ala-Ser22
Predicted Molecular Mass
19 kDa
SDS-PAGE
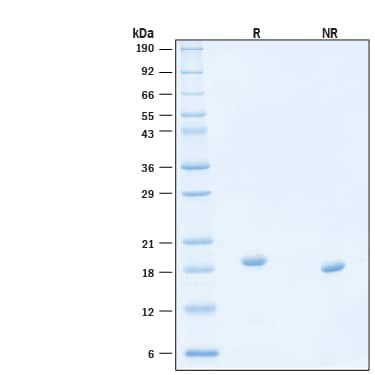
18-19 kDa, under reducing conditions.
Activity
Measured in a cell proliferation assay using MO7e human megakaryocytic leukemic cells. Avanzi, G. et al. (1988) Br. J. Haematol. 69:359.
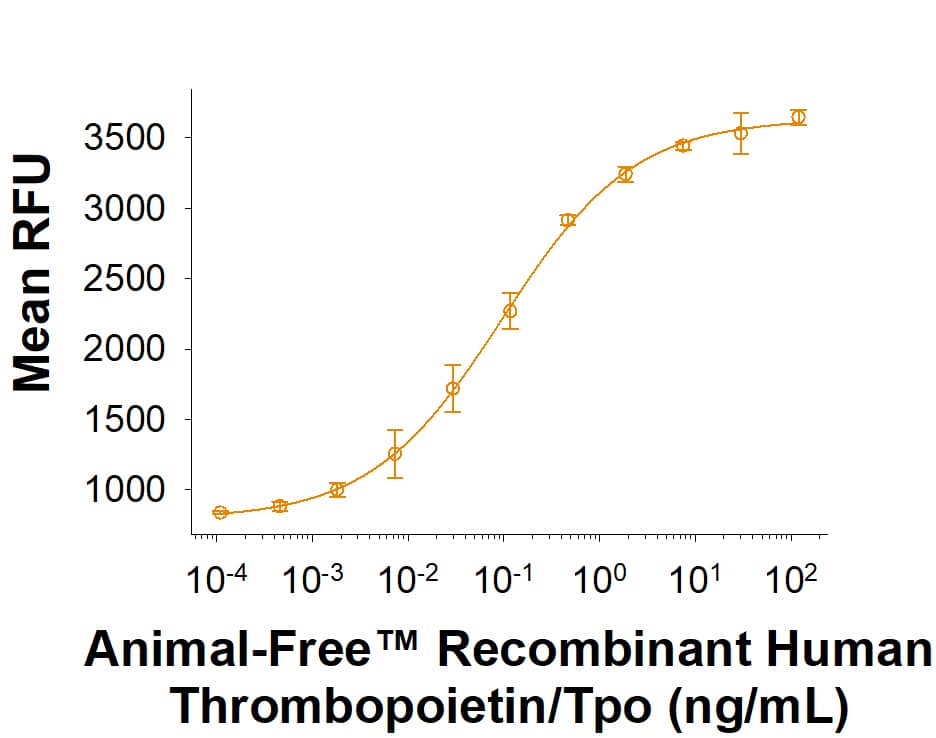
The ED50 for this effect is 0.050-0.500 ng/mL.
The specific activity of Recombinant Human Thrombopoietin is >1 x 107 units/mg, which is calibrated against the human Thrombopoietin reference standard (NIBSC code: 03/124).
The ED50 for this effect is 0.050-0.500 ng/mL.
The specific activity of Recombinant Human Thrombopoietin is >1 x 107 units/mg, which is calibrated against the human Thrombopoietin reference standard (NIBSC code: 03/124).
Scientific Data Images for Recombinant Human Thrombopoietin/TPO Protein, Animal-Free
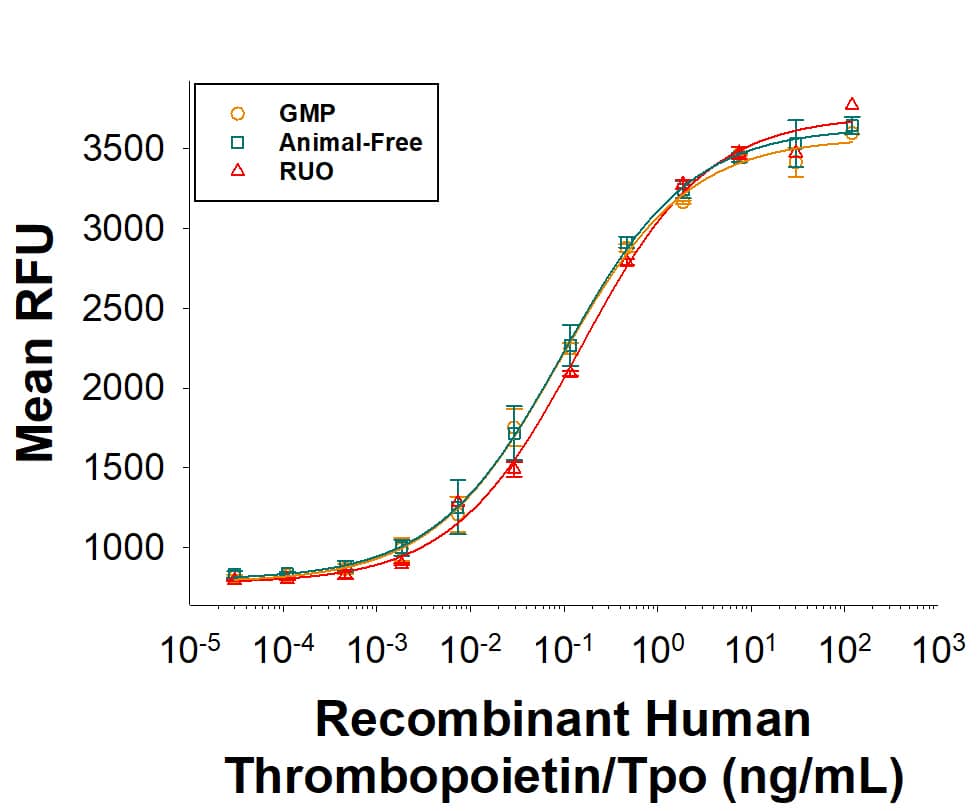
Equivalent Bioactivity of GMP, Animal-Free, and RUO grades of Recombinant Human Thrombopoietin/Tpo proteins.
Equivalent bioactivity of GMP (BT-TPO-GMP), Animal-Free (Catalog # BT-TPO-AFL) and RUO (BT-TPO) grades of Recombinant Human Thrombopoietin/Tpo proteins as measured in a cell proliferation assay using MO7e human megakaryocytic leukemic cells (orange, green, red, respectively).Animal-Free™ Recombinant Human Thrombopoietin/Tpo Protein Bioactivity.
Animal-Free™ Recombinant Human Thrombopoietin/Tpo (Catalog # BT-TPO-AFL) stimulates proliferation in the MO7e human megakaryocytic leukemic cell line. The ED50 for this effect is 0.050-0.500 ng/mL.Animal-Free™ Recombinant Human Thrombopoietin/Tpo Protein SDS-PAGE.
2 μg/lane of Animal-Free™ Recombinant Human Thrombopoietin/Tpo Protein (Catalog # BT-TPO-AFL) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 18-19 kDa, under reducing conditions.Formulation, Preparation and Storage
BT-TPO-AFL
| Formulation | Lyophilized from a 0.2 μm filtered solution in Acetonitrile and TFA with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in water. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Thrombopoietin/Tpo
Alternate Names
MGDF, MK-CSF, MKCSF, MPLLG, THCYT1, THPO, Tpo
Gene Symbol
THPO
UniProt
Additional Thrombopoietin/Tpo Products
Product Documents for Recombinant Human Thrombopoietin/TPO Protein, Animal-Free
Manufacturing Specifications
Animal-Free Manufacturing ConditionsOur dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
Purification
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers in a dedicated cold storage room.
- Low Endotoxin Level.
- No impairment of biological activity.
- High quality product obtained under stringent conditions.
- For ex vivo research or bioproduction, additional documentation can be provided.
Product Specific Notices for Recombinant Human Thrombopoietin/TPO Protein, Animal-Free
For research use or further manufacturing only
Loading...
Loading...
Loading...
Loading...