Human CTLA-4 Quantikine HS ELISA Kit
R&D Systems, part of Bio-Techne | Catalog # HSCT40

Key Product Details
Assay Length
Sample Type & Volume Required Per Well
Sensitivity
Assay Range
Product Summary for Human CTLA-4 Quantikine HS ELISA Kit
Product Specifications
Measurement
Detection Method
Conjugate
Reactivity
Specificity
Cross-reactivity
Interference
Precision
Intra-Assay Precision (Precision within an assay) Three samples of known concentration were tested twenty times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays) Three samples of known concentration were tested in twenty separate assays to assess inter-assay precision. Assays were performed by at least three technicians.
Cell Culture Supernates, Cell Lysates, EDTA Plasma, Heparin Plasma, Serum, Urine
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (pg/mL) | 5.53 | 15.6 | 32.1 | 5.32 | 14.7 | 31.2 |
| Standard Deviation | 0.191 | 0.409 | 0.528 | 0.331 | 0.851 | 1.98 |
| CV% | 3.5 | 2.6 | 1.6 | 6.2 | 5.8 | 6.3 |
Recovery for Human CTLA-4 Quantikine HS ELISA Kit
The recovery of human CTLA-4 spiked to levels throughout the range of the assay was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Media (n=4) | 105 | 102-108 |
| Cell Lysis Buffer (n=1) | 76 | 75-78 |
| EDTA Plasma (n=4) | 90 | 79-97 |
| Heparin Plasma (n=4) | 93 | 84-100 |
| Serum (n=4) | 94 | 85-102 |
| Urine (n=4) | 103 | 100-107 |
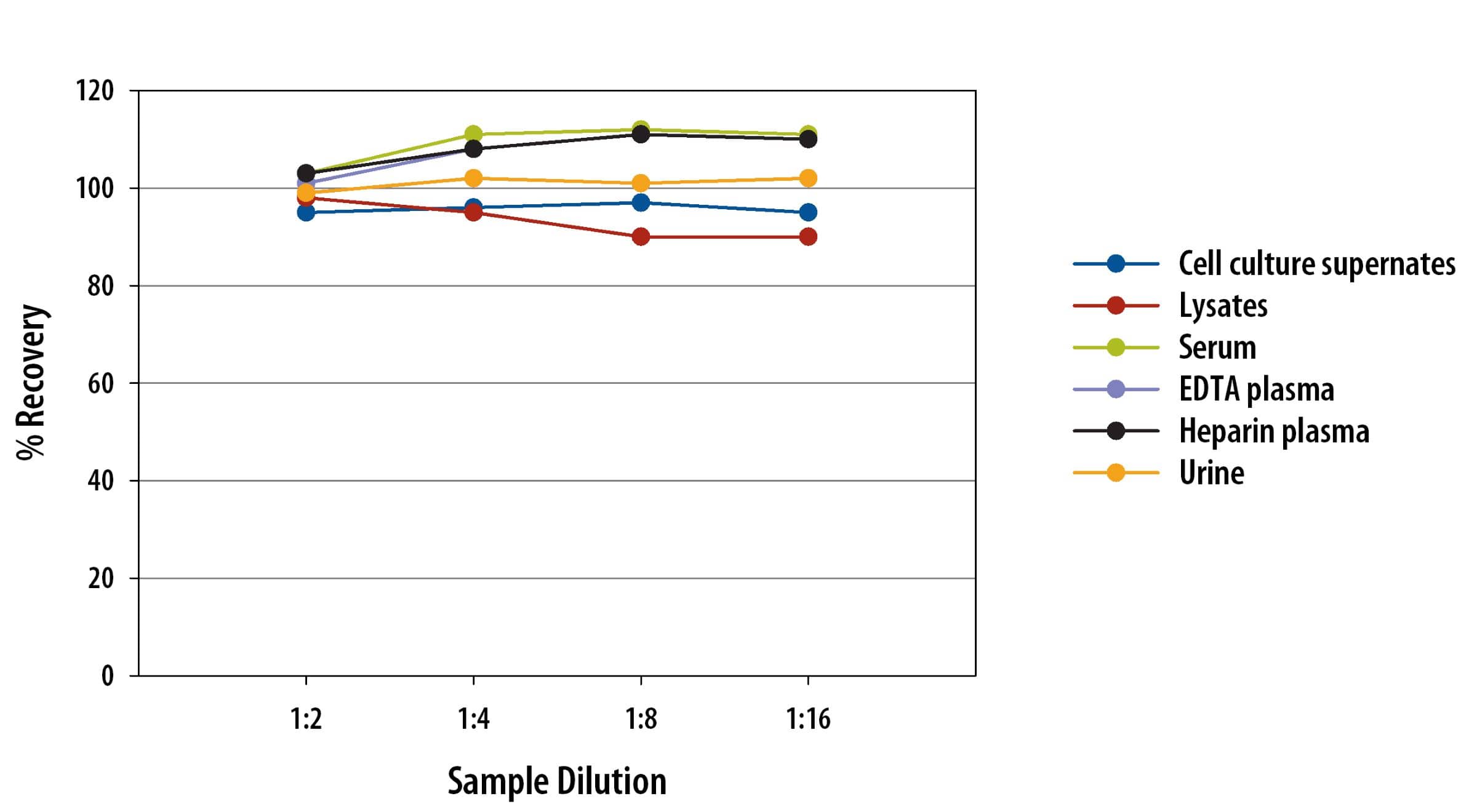
Linearity
To assess the linearity of the assay, samples containing and/or spiked with high concentrations of human CTLA-4 were serially diluted with calibrator diluent to produce samples with values within the dynamic range of the assay.
Scientific Data Images for Human CTLA-4 Quantikine HS ELISA Kit
Human CTLA-4 High Sensitivity ELISA Standard Curve
Preparation and Storage
Shipping
Stability & Storage
Background: CTLA-4
Long Name
Alternate Names
Gene Symbol
Additional CTLA-4 Products
Product Documents for Human CTLA-4 Quantikine HS ELISA Kit
Product Specific Notices for Human CTLA-4 Quantikine HS ELISA Kit
For research use only
