Detection of Mouse Podocalyxin Like by Immunocytochemistry/Immunofluorescence
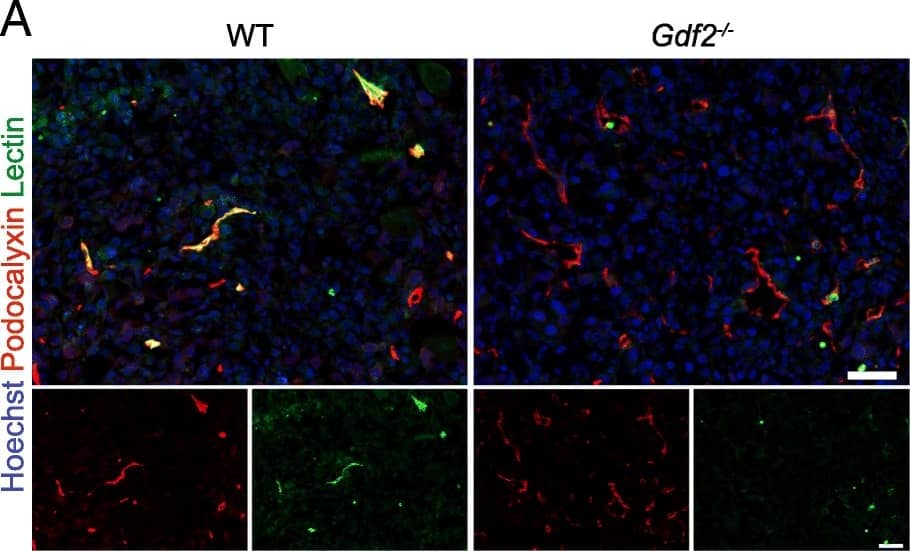
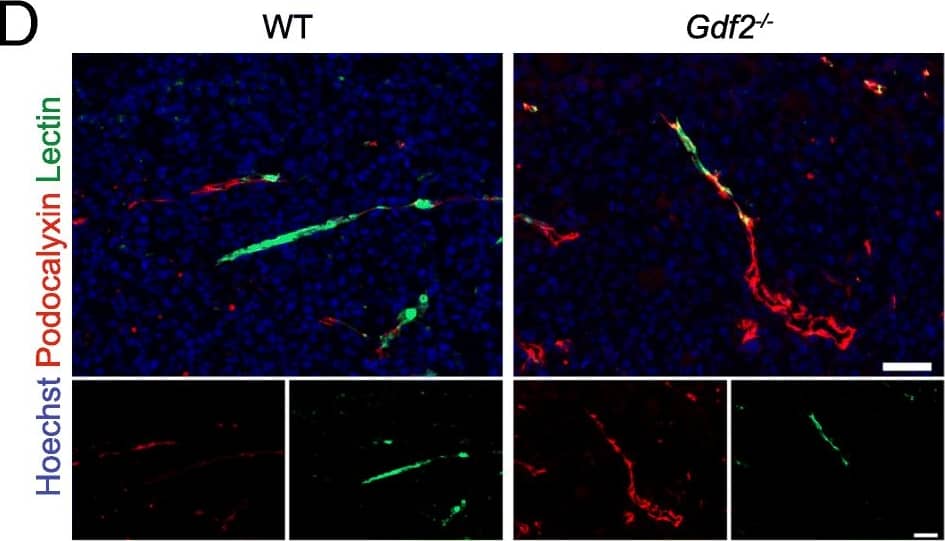
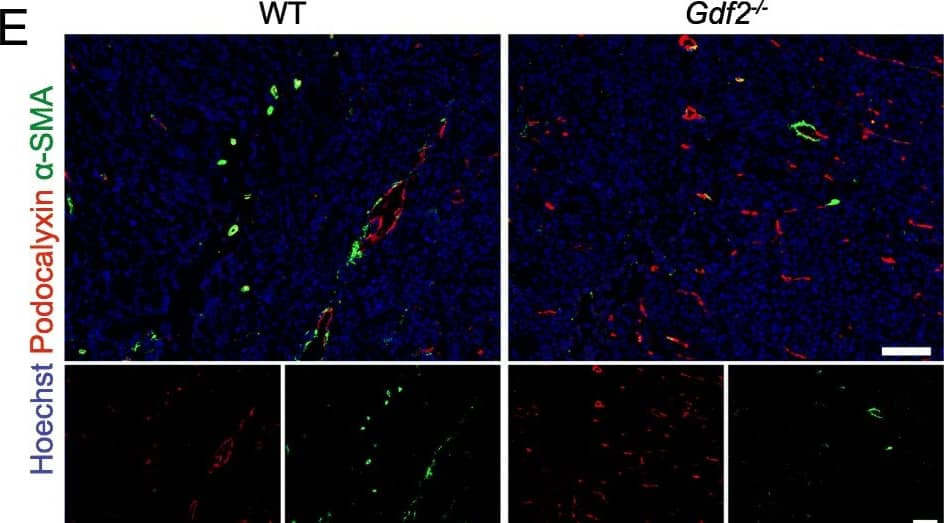
Gdf2 deletion decreases tumor perfusion and maturation in the E0771 mammary cancer model. E0771 cells were injected in the 4th mammary gland of WT and Gdf2−/− mice and tumor vascularization was analyzed 9 days after tumor detection. a Representative images of the tumors stained for podocalyxin (red), lectin (green) and cell nuclei (blue, Hoechst). Scale bar 50 μm. b Vascular density quantified by podocalyxin positive area (% of tumor area) and (c) assessment of vessel diameter using Ferret’s theorem (WT n = 7, Gdf2−/−n = 13, 1 representative experiment out of 2). d Quantification of vessel perfusion by lectin staining (% area of lectin/podocalyxin) (WT n = 8, Gdf2−/− n = 7, 1 representative experiment out of 3). e Representative images of the tumors stained for podocalyxin (red), alpha-smooth muscle actin ( alpha-SMA) (green) and cell nuclei (blue, Hoechst). Scale bar 100 μm. f alpha-SMA staining quantification (% area of alpha-SMA/podocalyxin) (WT n = 8, Gdf2−/− n = 7, 1 representative experiment out of 3). b, c, d, f Data are the median ± interquartile range. Statistical analysis: Mann-Whitney test. *p ≤ 0.05 and **p ≤ 0.01 significantly different Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30165893), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Podocalyxin Like by Immunocytochemistry/Immunofluorescence
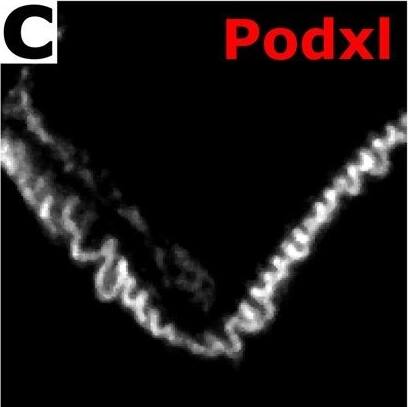
Confocal ExM images of mouse kidney labeled with antibodies or fluorescent proteins. (a–c) Single focal plane of glomerulus immunostained for podocin (a), agrin (b), podocalyxin (Podxl c), and merge (d) of (a–c). (e–g) Confocal maximum intensity projections of glomerulus immunostained for synaptopodin (Synpo, e), acetylated tubulin (acTub, f), podocin (g) highlighting secondary FPs, primary FPs, and slit diaphragms/FP boundaries, respectively. (h) Merge of (e–h). (i–k) Confocal maximum intensity projections of glomerulus immunostained for collagen IV (Coll IV, i), podocalyxin (Podxl, j), and alpha smooth muscle actin ( alphaSMA, k) and highlighting Bowman’s capsule and the mesangium, podocytes, and arterioles and the mesangium, respectively. (l) Merge of (i–k). (m–p) Single focal plane of glomerulus showing native fluorescence from confetti mouse expressing YFP (m) and RFP (o) in separate podocyte cell bodies and FPs as well as GFP (n) in various podocyte nuclei. (p) Merge of (m–o). (q) Zoomed-in view of region highlighted in (p). (r) Further zoomed-in view (top) and cross-sectional profile (bottom) of boxed region highlighted in (q). All distances and scale bars are in pre-expansion units. Scale bars, 2 µm (a–h,q), 25 µm (i–l), 5 µm (m–p). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29991751), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Podocalyxin Like by Immunocytochemistry/Immunofluorescence
Endothelial beta-catenin GOF does not affect the ECM of astrocytic endfeet and ECs within the subfornical organ (SFO).Striatal BBB-vessel showing a polarized distribution of Lama2 and Aqp4 in AC endfeet. Lumen is stained by Podxl (asterisk) (A). Coronal overview of the subfornical organ (SFO) (B); rectangular inset demarcates area for higher magnification in (C). Striatal BBB-vessel showing a polarized distribution of ColIV (green) but no Meca32 (white) in ECs (D). Coronal overview SFO, rectangular inset demarcates area for higher magnification in F (E); white dashed lines show Meca32+, red dashed lines show Meca32 vessels (F). Dashed lines outline SFO vessels; scale bars show 2 µm (A), 50 µm (B), 10 µm (C), 2.5 µm (D), 50 µm (E), 10 µm (F). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30932814), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Podocalyxin Like by Immunocytochemistry/Immunofluorescence
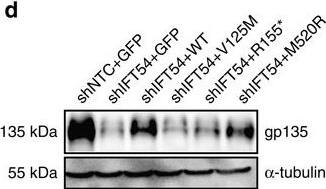
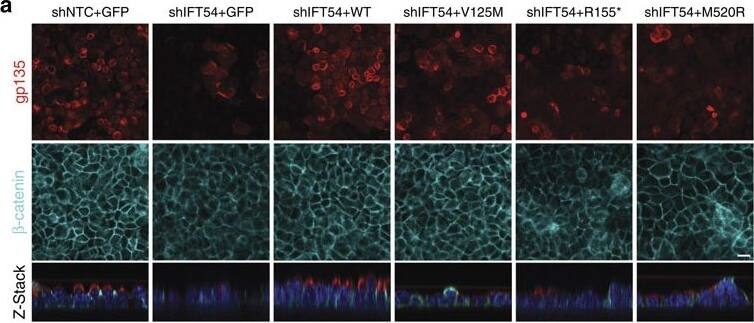
TRAF3IP1 mutations lead to epithelialization and polarity defects.(a) mIMCD3 cells grown until confluence on filters were subjected to Ca2+-free medium to disrupt the tight junctions. Six hours after Ca2+ addition, cells were analysed by immunofluorescence using the apical marker Gp135 (red) and beta-catenin (light blue) to stain the cell junctions. Scale bar, 10 μm. (b) Following Ca2+ switch, tight junction re-formation was assessed by measurement of trans-epithelial resistance (TER) at different time points (mean ± s.e.m. of n=5 independent experiments, two-way ANOVA; NS: not-significant, ***P<0.001 at 6 h). (c) Height of mIMCD3 cells grown on filters measured as the distance from the base to the top of the cells (GFP staining, not shown; mean ± s.d. of n≥20, from 3 independent experiments, ***P<0.001, Bonferonni's multiple-comparison test). (d) Expression of the apical marker Gp135 was analysed by Western blot with alpha-tubulin as a loading control. (e) mIMCD3 cells grown in matrigel 3D matrix to form spheroids were stained for ZO1 (tight junctions, red) and analysed by confocal microscopy. Arrows indicate ZO-1 at the apical junctions, while arrow heads point to mislocalized ZO-1. Equatorial sections of representative spheres are shown for each cell line. Scale bars, 10 μm. (f) Percentage of abnormal spheroids (no/small lumen filled with cells) (mean ± s.d., n=80 spheroids from 2 independent experiments, ***P≤0,001, **P<0.002, Bonferonni's multiple-comparison test). Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/ncomms9666), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Podocalyxin Like by Immunocytochemistry/Immunofluorescence
Bmp10 conditional deletion has no impact on tumor growth, angiogenesis and lung metastasis in the E0771 mammary cancer model. a Schematic representation of the experimental protocol for Bmp10 specific deletion and E0771 cells implantation. Tamoxifen was injected in all 3-week-old mice; 3 weeks later, E0771 cells were injected and tumor growth was analyzed for 3 weeks. b Plasmatic levels of BMP10 in control (CTL, n = 15) and Bmp10 conditional KO (Bmp10-cKO, n = 15) mice assessed by ELISA at the end of the experiment. c Tumor growth was assessed by caliper measurement every 2 to 3 days after tumor detection (CTL n = 7, Bmp10-cKO n = 8, 1 representative experiment out of 3). d Representative images of the tumors stained for podocalyxin (red), lectin (green) and cell nuclei (blue, Hoechst). Scale bar 50 μm. e Vascular density quantified by podocalyxin surface area (% of tumor area) and (f) Quantification of vessel perfusion by lectin staining (% area of lectin/podocalyxin) (CTL n = 7, Bmp10-cKO n = 8, 1 representative experiment out of 3). g Total area, (h) number and (i) mean size of lung metastases per mice bearing metastases (CTL n = 10, Bmp10-cKO n = 9, 2 experiments). c Data are the mean ± SEM. Statistical analysis: Two-way matched ANOVA. b, e, f, g, h, i Data are the median ± interquartile range. Statistical analysis: Mann-Whitney test. ****p ≤ 0.001 significantly different Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30165893), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Podocalyxin Like by Immunocytochemistry/Immunofluorescence
Endothelial beta-catenin GOF does not affect astrocytic endfoot polarization of alpha-dystroglycan ( alpha-Dag) and Kir4.1 within the subfornical organ (SFO).Striatal BBB-vessel showing a polarized distribution of alpha-Dag and Kir4.1 in AC endfeet. Lumen is stained by Podxl (asterisk) (A). Coronal overview of the subfornical organ (SFO) (B); rectangular inset demarcates area for higher magnification in (C). Dashed lines outline SFO vessels. Scale bar show 2 µm (A), 50 µm (B), 10 µm (C). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30932814), licensed under a CC-BY license. Not internally tested by R&D Systems.
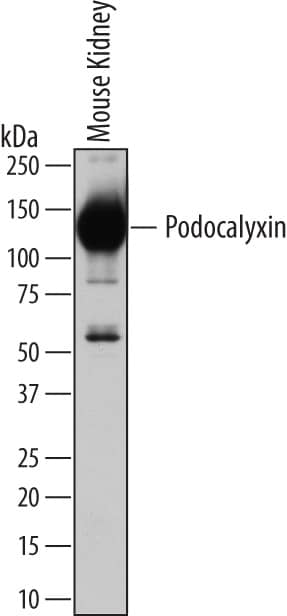
Detection of Mouse Podocalyxin Like by Western Blot
TRAF3IP1 mutations lead to epithelialization and polarity defects.(a) mIMCD3 cells grown until confluence on filters were subjected to Ca2+-free medium to disrupt the tight junctions. Six hours after Ca2+ addition, cells were analysed by immunofluorescence using the apical marker Gp135 (red) and beta-catenin (light blue) to stain the cell junctions. Scale bar, 10 μm. (b) Following Ca2+ switch, tight junction re-formation was assessed by measurement of trans-epithelial resistance (TER) at different time points (mean ± s.e.m. of n=5 independent experiments, two-way ANOVA; NS: not-significant, ***P<0.001 at 6 h). (c) Height of mIMCD3 cells grown on filters measured as the distance from the base to the top of the cells (GFP staining, not shown; mean ± s.d. of n≥20, from 3 independent experiments, ***P<0.001, Bonferonni's multiple-comparison test). (d) Expression of the apical marker Gp135 was analysed by Western blot with alpha-tubulin as a loading control. (e) mIMCD3 cells grown in matrigel 3D matrix to form spheroids were stained for ZO1 (tight junctions, red) and analysed by confocal microscopy. Arrows indicate ZO-1 at the apical junctions, while arrow heads point to mislocalized ZO-1. Equatorial sections of representative spheres are shown for each cell line. Scale bars, 10 μm. (f) Percentage of abnormal spheroids (no/small lumen filled with cells) (mean ± s.d., n=80 spheroids from 2 independent experiments, ***P≤0,001, **P<0.002, Bonferonni's multiple-comparison test). Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/ncomms9666), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Podocalyxin Like by Immunocytochemistry/Immunofluorescence
Gdf2 deletion decreases tumor perfusion and maturation in the E0771 mammary cancer model. E0771 cells were injected in the 4th mammary gland of WT and Gdf2−/− mice and tumor vascularization was analyzed 9 days after tumor detection. a Representative images of the tumors stained for podocalyxin (red), lectin (green) and cell nuclei (blue, Hoechst). Scale bar 50 μm. b Vascular density quantified by podocalyxin positive area (% of tumor area) and (c) assessment of vessel diameter using Ferret’s theorem (WT n = 7, Gdf2−/−n = 13, 1 representative experiment out of 2). d Quantification of vessel perfusion by lectin staining (% area of lectin/podocalyxin) (WT n = 8, Gdf2−/− n = 7, 1 representative experiment out of 3). e Representative images of the tumors stained for podocalyxin (red), alpha-smooth muscle actin ( alpha-SMA) (green) and cell nuclei (blue, Hoechst). Scale bar 100 μm. f alpha-SMA staining quantification (% area of alpha-SMA/podocalyxin) (WT n = 8, Gdf2−/− n = 7, 1 representative experiment out of 3). b, c, d, f Data are the median ± interquartile range. Statistical analysis: Mann-Whitney test. *p ≤ 0.05 and **p ≤ 0.01 significantly different Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30165893), licensed under a CC-BY license. Not internally tested by R&D Systems.
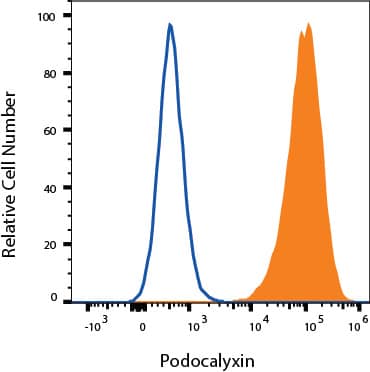
Detection of Podocalyxin in Neuro-2A cells by Flow Cytometry
Neuro-2A cells were stained with Goat Anti-Mouse Podocalyxin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1556, filled histogram) or isotype control antibody (Catalog # 4-001-A, open histogram) followed by Allophycocyanin-conjugated Anti-Goat IgG Secondary Antibody (Catalog #
F0108). View our protocol for Staining Membrane-associated Proteins.
Detection of Mouse Mouse Podocalyxin Antibody by Immunohistochemistry
TRAF3IP1 mutations lead to epithelialization and polarity defects.(a) mIMCD3 cells grown until confluence on filters were subjected to Ca2+-free medium to disrupt the tight junctions. Six hours after Ca2+ addition, cells were analysed by immunofluorescence using the apical marker Gp135 (red) and beta-catenin (light blue) to stain the cell junctions. Scale bar, 10 μm. (b) Following Ca2+ switch, tight junction re-formation was assessed by measurement of trans-epithelial resistance (TER) at different time points (mean ± s.e.m. of n=5 independent experiments, two-way ANOVA; NS: not-significant, ***P<0.001 at 6 h). (c) Height of mIMCD3 cells grown on filters measured as the distance from the base to the top of the cells (GFP staining, not shown; mean ± s.d. of n≥20, from 3 independent experiments, ***P<0.001, Bonferonni's multiple-comparison test). (d) Expression of the apical marker Gp135 was analysed by Western blot with alpha-tubulin as a loading control. (e) mIMCD3 cells grown in matrigel 3D matrix to form spheroids were stained for ZO1 (tight junctions, red) and analysed by confocal microscopy. Arrows indicate ZO-1 at the apical junctions, while arrow heads point to mislocalized ZO-1. Equatorial sections of representative spheres are shown for each cell line. Scale bars, 10 μm. (f) Percentage of abnormal spheroids (no/small lumen filled with cells) (mean ± s.d., n=80 spheroids from 2 independent experiments, ***P≤0,001, **P<0.002, Bonferonni's multiple-comparison test). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26487268), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Podocalyxin Like by Immunohistochemistry
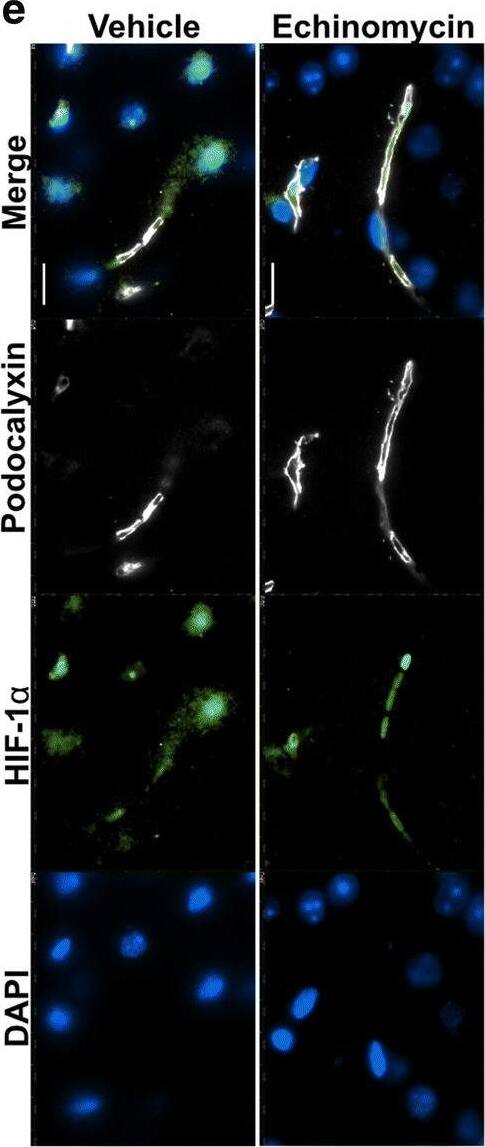
Improved survival&blood–brain barrier function post pneumococcal infection in mice by anti-HIF-1 alpha treatment using echinomycin. e Immunofluorescence staining for HIF-1 alpha, BBB permeability&junctional markers in the echinomycin&vehicle groups at the survival end point. Left panel shows that echinomycin treatment leads to a reduction in HIF-1 alpha-positive nuclei including in ECs co-stained for podocalyxin, a vascular marker which was unchanged by the treatment. Middle panel displays reduced vascular permeability to fibrinogen in the echinomycin group as indicated by stronger intravascular signal compared to the vehicle-treated mice. Increased expression of tight junction proteins—occludin (middle panel)&claudin-5 (right top panel)—in echinomycin-treated mice indicate improved BBB function. Tight junction-associated ZO-1, adherens junction marker VE-Cadherin,&endothelial cell adhesion molecule CD31 unchanged (middle, bottom right panel). There was also no difference in S. pneumoniae (Spn) staining (right top panel) between the two groups. Scale bar 10 μm. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/32529267), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Podocalyxin by Western Blot
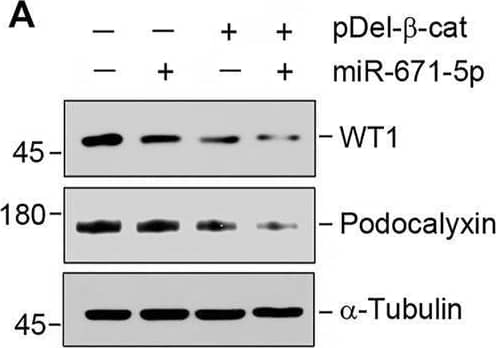
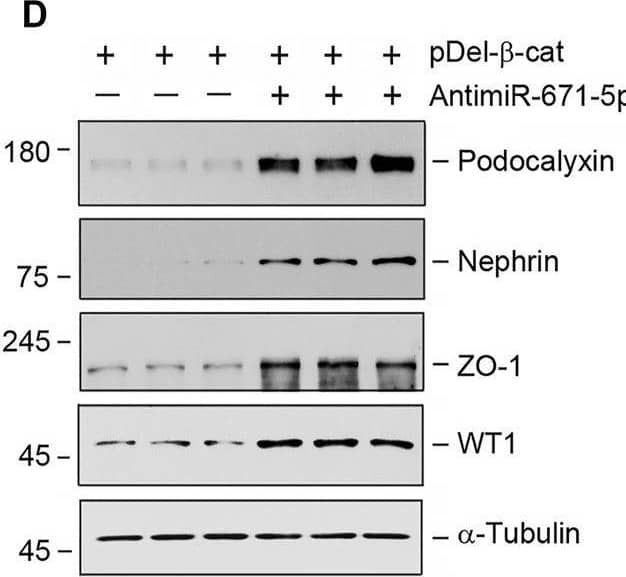
miR-671-5p aggravates beta-catenin-induced podocyte injury while miR-671-5p inhibitor ameliorates it in vitro. (A–C) Representative Western blot (A) and graphic presentations of WT1 (B) and podocalyxin (C) were presented. MPC5 cells were transfected with miR-671-5p mimics (miR-671-5p) or/and beta-catenin expression plasmid (pDel-beta -cat) for 24 h *p < 0.05 versus pcDNA3 controls; †p < 0.05 versus pDel-beta -cat (n = 3). (D–H) Representative Western blot (D) and graphic presentations of podocalyxin (E), nephrin (F), ZO-1 (G) and WT1 (H) were presented. MPC5 cells were transfected with beta-catenin expression plasmid (pDel-beta -cat) or/and miR-671-5p inhibitor (AntimiR-671-5p) for 24 h *p < 0.05 (n = 3). (I) Representative micrographs show the expression of ZO-1 in different groups as indicated. MPC5 cells were transfected with beta-catenin expression plasmid (pDel-beta -cat) and miR-671-5p mimics (miR-671-5p)/miR-671-5p inhibitor (AntimiR-671-5p) for 24 h, respectively. Scale bar, 50 µm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35111054), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Podocalyxin by Western Blot
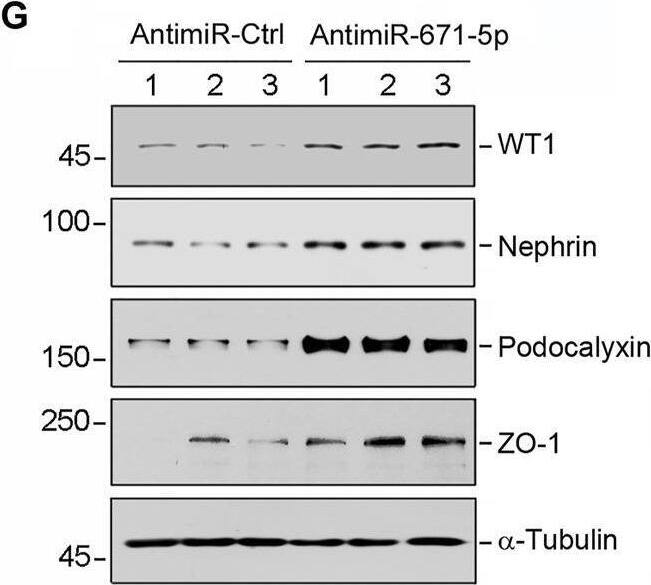
Overexpression of miR-671-5p impairs but knockdown of miR-671-5p protects podocyte integrity in vitro. Mouse podocytes (MPC5) were transfected with miR-671-5p mimics (miR-671-5p) or negative control (miR-Ctrl) for 24 h. (A) qRT-PCR analysis shows the relative levels of miR-671-5p after transfection. *p < 0.05 (n = 3). (B–E) Representative Western blot (B) and graphic presentations of WT1 (C), ZO-1 (D) and podocalyxin (E) were presented. *p < 0.05 (n = 3). (F) Representative micrographs show the expression and distribution of ZO-1 in podocytes after miR-671-5p overexpression. Scale bar, 50 µm. (G–K) Inhibition of miR-671-5p protects podocyte integrity. MPC5 cells were transfected with miR-671-5p inhibitor (AntimiR-671-5p) or control (AntimiR-Ctrl) or for 24 h. Representative Western blot (G) and graphic presentations of WT1 (H), nephrin (I), podocalyxin (J) and ZO-1 (K) were presented. *p < 0.05 (n = 3). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35111054), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Podocalyxin by Western Blot
miR-671-5p aggravates beta-catenin-induced podocyte injury while miR-671-5p inhibitor ameliorates it in vitro. (A–C) Representative Western blot (A) and graphic presentations of WT1 (B) and podocalyxin (C) were presented. MPC5 cells were transfected with miR-671-5p mimics (miR-671-5p) or/and beta-catenin expression plasmid (pDel-beta -cat) for 24 h *p < 0.05 versus pcDNA3 controls; †p < 0.05 versus pDel-beta -cat (n = 3). (D–H) Representative Western blot (D) and graphic presentations of podocalyxin (E), nephrin (F), ZO-1 (G) and WT1 (H) were presented. MPC5 cells were transfected with beta-catenin expression plasmid (pDel-beta -cat) or/and miR-671-5p inhibitor (AntimiR-671-5p) for 24 h *p < 0.05 (n = 3). (I) Representative micrographs show the expression of ZO-1 in different groups as indicated. MPC5 cells were transfected with beta-catenin expression plasmid (pDel-beta -cat) and miR-671-5p mimics (miR-671-5p)/miR-671-5p inhibitor (AntimiR-671-5p) for 24 h, respectively. Scale bar, 50 µm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35111054), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Podocalyxin by Western Blot
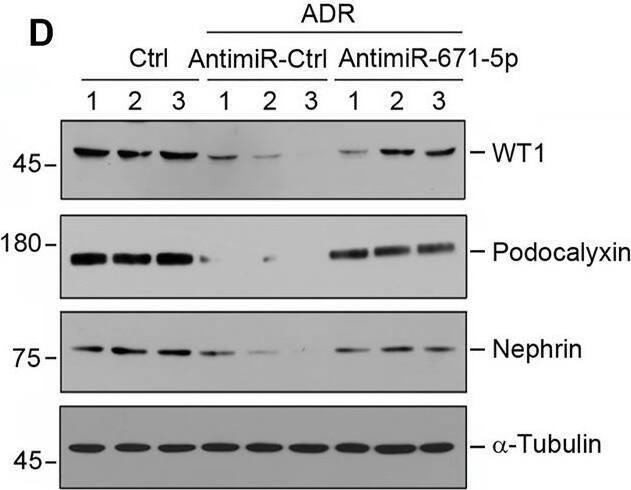
Inhibition of miR-671-5p reduces proteinuria and renal fibrotic lesions in ADR nephropathy. (A) Experimental design. Red Arrows indicate the time of ADR injection. Green arrows indicate the different time points of antagomir injections. (B) qRT-PCR analysis shows that miR-671-5p level was increased in ADR group compared with control, and injections of antimiR-671-5p decreased miR-671-5p level. *p < 0.05 versus normal controls; †p < 0.05 versus ADR (n = 5–6). (C) Inhibition of miR-671-5p reduces proteinuria in ADR nephropathy. Urinary albumin levels were assessed in mice at 2 weeks after ADR injection and expressed as mg/mg creatinine. *p < 0.05 versus normal controls; †p < 0.05 versus ADR (n = 5–6). (D–G) Representative Western blots (D) and graphic presentations of WT1 (E), podocalyxin (F) and nephrin (G) were presented. *p < 0.05 versus normal controls, †p < 0.05 versus ADR alone (n = 5–6). (H) Immunofluorescence staining shows that antimiR-671-5p preserved renal podocalyxin expression in ADR nephropathy. Arrow indicate positive staining. Scale bar, 20 µm. (I,J) Representative Western blots (I) and graphic presentations of fibronectin and alpha-SMA (J) were presented. *p < 0.05 versus normal controls, †p < 0.05 versus ADR alone (n = 5–6). (K) Representative micrographs show that antimiR-671-5p inhibited alpha-SMA expression (upper panel) and renal fibrotic lesions (bottom panel) in different groups as indicated. Scale bar, 50 µm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35111054), licensed under a CC-BY license. Not internally tested by R&D Systems.