Memory T Cell Subset Phenotype Flow Cytometry Panel
The frequency of different memory T cell subsets in a sample can dramatically impact the expansion potential and functionality of a host immune response. Use this validated multicolor flow cytometry panel to phenotype human PBMCs for memory T cell subsets, including Naïve T cells (Tn), Central Memory (Tcm), and Effector Memory (Tem).
Flow Cytometry Panel for Immunophenotyping of Memory T Cell Subsets
| Marker | Clone | Fluorochrome | Catalog # |
| CD3 | UCHT1* | mFluor™ Violet 450 | FAB100MFV450 |
| CD4 | 11830 | mFluor™ Violet 500 | FAB3791MFV500 |
| CD8 | 37006 | Alexa Fluor® 700 | FAB1509N |
| Live/Dead | (APC-Cy7) | ||
| CD45RA | 1069433 | mFluor™ Violet 610 | FAB11444MFV610 |
| CD45RO | UCHL1 | Alexa Fluor® 647 | FAB10642R |
| CCR7 | 150503 | PE | FAB197P |
*Designate clones independently validated by HLDA.
Alexa Fluor® is registered trademark of Molecular Probes, Inc.
mFluor is a trademark of AAT Bioquest.
This multicolor flow cytometry panel was validated on human peripheral blood mononuclear cells (PBMCs).
Flow Cytometry Gating Strategy for Memory T Cell Panel
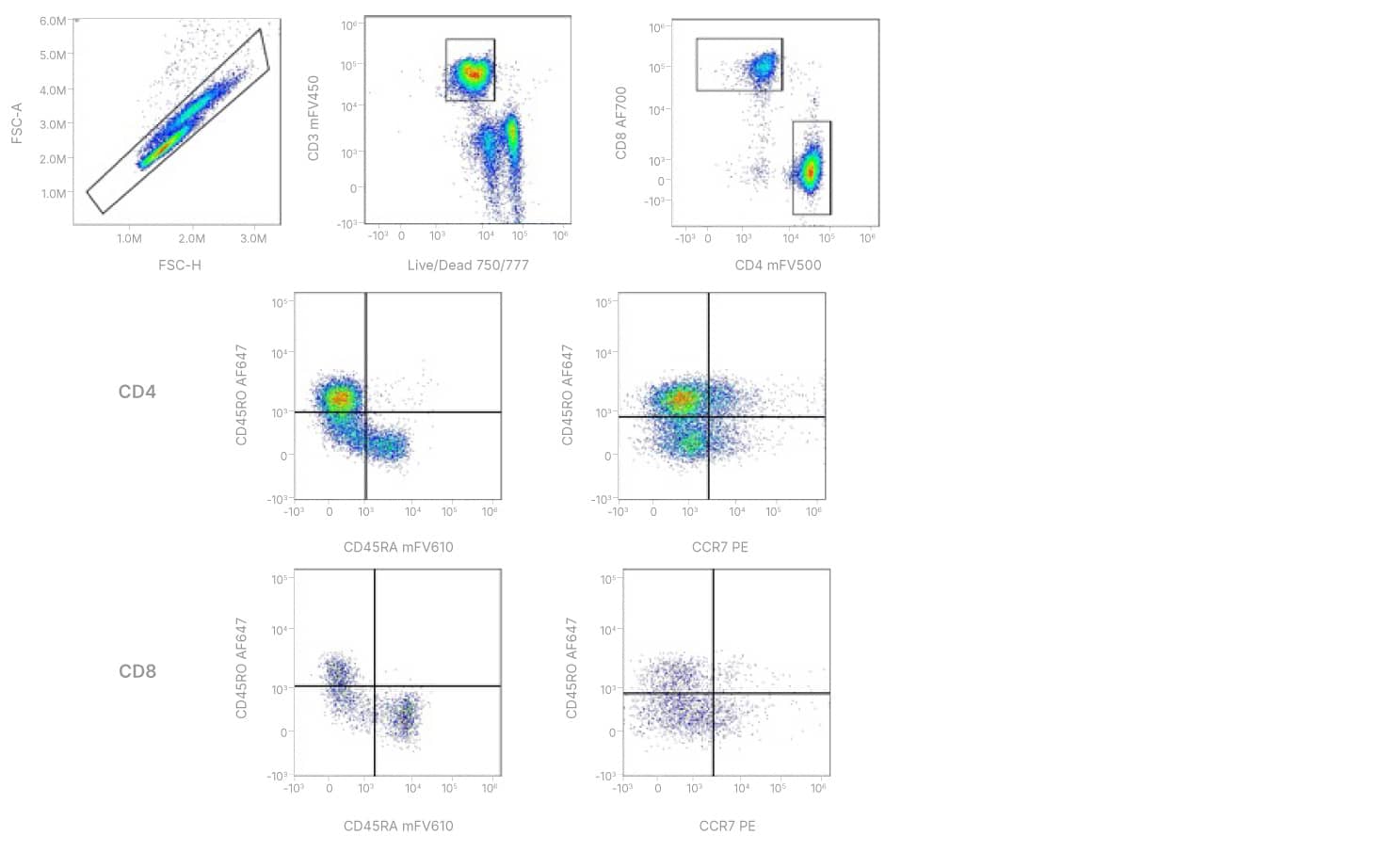
Multicolor flow cytometry panel to identify human Memory T cell subsets. Naïve PBMCs were stained with anti-human CD3 mFluor™ Violet 450, CD4 mFluor™ Violet 500, CD8 Alexa Fluor® 700, CCR7 PE, CD45RO Alexa Fluor® 647, and CD45RA mFluor™ Violet 610. Gating strategy: Single Cells/Viable CD3+ cells/CD4+ vs. CD8+ cells. CD45RO, CD45RA, and CCR7 analysis was carried out on CD4+ and CD8+ subsets.
Staining Protocol For T Cell Memory Panel
Other supplies required
- PBS
- Flow Cytometry Staining Buffer (Catalog # FC001)
- Fc-block (blocking IgG)
- (Optional) Isotype Control Antibodies
- 5 mL Flow cytometry tubes
1. Wash human PBMCs (1 x 106 cells per sample) with 2 mL of Staining Buffer (1X) (Catalog # FC001) or other BSA-containing buffer, by spinning at 300 x g for 5 minutes, using 5 mL flow cytometry tubes. Decant/aspirate supernatant.
2. Fc-block cells with blocking IgG (1 μg IgG/106 cells) for 10 minutes at room temperature.
3. Add previously titrated amount of each primary conjugated antibody. Vortex tubes.
| Marker | Fluorochrome | Volume/test (µL) |
| CD3 | mFluor™ Violet 450 | 5 |
| CD4 | mFluor™ Violet 500 | 5 |
| CD8 | Alexa Fluor® 700 | 5 |
| Live/Dead | (APC-Cy7) | 0.1 |
| CD45RA | mFluor™ Violet 610 | 5 |
| CD45RO | Alexa Fluor® 647 | 5 |
| CCR7 | PE | 10 |
4. (Optional) To a separate tube, add 5 μL of each of the isotype control antibodies. Vortex tubes.
5. Incubate the mixtures for 30-45 minutes at room temperature in the dark.
6. At the end of the incubation, wash with 2 mL of Staining Buffer (1X), by spinning at 300 x g for 5 minutes. Decant/aspirate supernatant.
Additional Flow Cytometry Products and Resources
Products:
MagCellect™ Cell Selection Kits
Quality Control and Standardization Beads from Novus Biologicals
Resources:
Intracellular Staining with Alcohol Permeabilization Protocol
Intracellular Staining with Detergent Permeabilization Protocol
On-Demand Webinar: Demystifying Multi-parameter Flow Cytometry
On Demand Webinar: Turning Flow Cytometry Upside Down and Inside Out