Recombinant Human Sonic Hedgehog/Shh (C24II) N-Terminus Best Seller
R&D Systems, part of Bio-Techne | Catalog # 1845-SH

Key Product Details
Product Specifications
Source
Cys24-Gly197 (Cys24Ile-Ile), with an N-terminal Met
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
SDS-PAGE
Activity
The ED50 for this effect is 0.1-0.4 µg/mL.
Reviewed Applications
Read 5 reviews rated 4.8 using 1845-SH in the following applications:
Scientific Data Images for Recombinant Human Sonic Hedgehog/Shh (C24II) N-Terminus
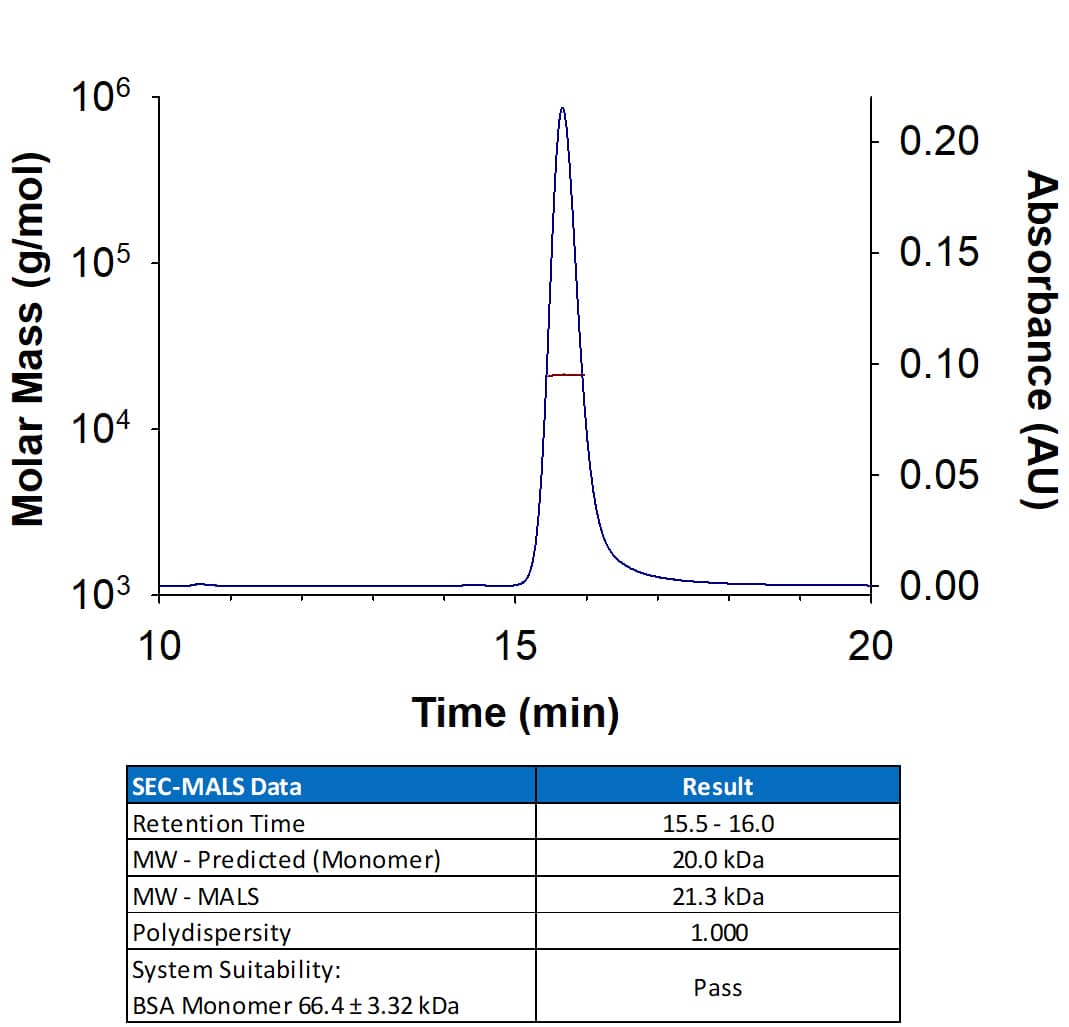
Recombinant Human Sonic Hedgehog/Shh (C24II), N-Terminus Protein SEC-MALS.
Recombinant Human SHH Protein (Catalog # 1845-SH) has a molecular weight (MW) of 21.3 kDa as analyzed by SEC-MALS, suggesting that this protein is a monomer.Recombinant Human Sonic Hedgehog/Shh (C24II) N-Terminus Bioactivity
Recombinant Human Sonic Hedgehog/Shh (C24II), N-Terminus (Catalog # 1845-SH) induces alkaline phosphatase production by the C3H10T1/2 mouse embryonic fibroblast cell line. The ED50 for this effect is 0.1-0.4 μg/mL.Recombinant Human Sonic Hedgehog/Shh (C24II) N-Terminus SDS-PAGE
1 μg/lane of Recombinant Human Sonic Hedgehog/Shh (C24II), N-Terminus was resolved with SDS-PAGE under reducing (R) conditions and visualized by silver staining, showing a single band at 22 kDa.Formulation, Preparation and Storage
Carrier Free
What does CF mean?CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
What formulation is right for me?In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
Carrier: 1845-SH
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS and NaCl with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100-200 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin, and allow up to 24 hours at 2 to 8 °C for complete reconstitution. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Carrier Free: 1845-SH/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS and NaCl. |
| Reconstitution | Reconstitute at 100-200 μg/mL in sterile PBS, and allow up to 24 hours at 2 to 8 °C for complete reconstitution. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Sonic Hedgehog/Shh
N-terminal cysteine and modified by cholesterol addition at its C-terminus (6). These modifications contribute to the membrane tethering of Shh as well as its assembly into various sized multimers (6-9). Lipid modification and multimerization greatly increase Shh-N receptor binding affinity and signaling potency (5, 6, 8, 9). Monomeric and multimeric Shh can be released from the plasma membrane by the cooperative action of DISP1, SCUBE2, and TACE/ADAM17 (10-12). Modifications also extend the effective range of Shh functionality and are required for the development of protein gradients important in tissue morphogenesis (9, 13). Canonical signaling of Shh is mediated by a multicomponent receptor complex that includes Patched (PTCH1, PTCH2) and Smoothened (SMO) (14). The binding of Shh to PTCH releases the basal repression of SMO by PTCH. Shh activity can also be regulated through interactions with heparin, glypicans, and membrane-associated Hip (hedgehog interacting protein) (13, 15, 16).
References
- Briscoe, J. and P.P. Therond (2013) Mol. Cell. Biol. 14:416.
- Aviles, E.C. et al. (2013) Front. Cell. Neurosci. 7:86.
- Xie, J. et al. (2013) OncoTargets Ther. 6:1425.
- Marigo, V. et al. (1995) Genomics 28:44.
- Zeng, X. et al. (2001) Nature 411:716.
- Feng, J. et al. (2004) Development 131:4357.
- Goetz, J.A. et al. (2006) J. Biol. Chem. 281:4087.
- Pepinsky, R.B. et al. (1998) J. Biol. Chem. 273:14037.
- Chen, M.-H. et al. (2004) Genes Dev. 18:641.
- Etheridge, L.A. et al. (2010) Development 137:133.
- Jakobs, P. et al. (2014) J. Cell Sci. 127:1726.
- Dierker, T. et al. (2009) J. Biol. Chem. 284:8013.
- Lewis, P.M. et al. (2001) Cell 105:599.
- Carpenter, D. et al. (1998) Proc. Natl. Acad. Sci. USA 95:13630.
- Filmus, J. and M. Capurro (2014) Matrix Biol. 35:248.
- Chuang, P.-T. and A.P. McMahon (1999) Nature 397:617.
Alternate Names
Gene Symbol
UniProt
Additional Sonic Hedgehog/Shh Products
Product Documents for Recombinant Human Sonic Hedgehog/Shh (C24II) N-Terminus
Product Specific Notices for Recombinant Human Sonic Hedgehog/Shh (C24II) N-Terminus
For research use only