Recombinant Human LAIR1 His-tag Avi-tag Protein, CF
R&D Systems, part of Bio-Techne | Catalog # AVI2664

Key Product Details
- R&D Systems HEK293-derived Recombinant Human LAIR1 His-tag Avi-tag Protein (AVI2664)
- Quality control testing to verify active proteins with lot specific assays by in-house scientists
- All R&D Systems proteins are covered with a 100% guarantee
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
Source
| Human LAIR1 (Gln22-His163) Accession # NP_002278.2 |
HHHHHH | Avi-tag |
| N-terminus | C-terminus |
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
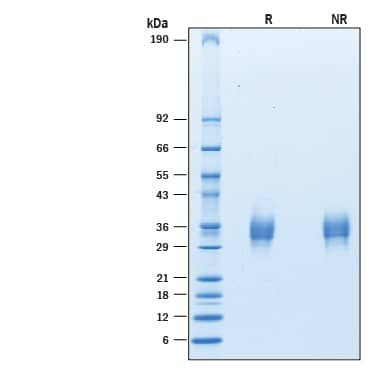
SDS-PAGE
Activity
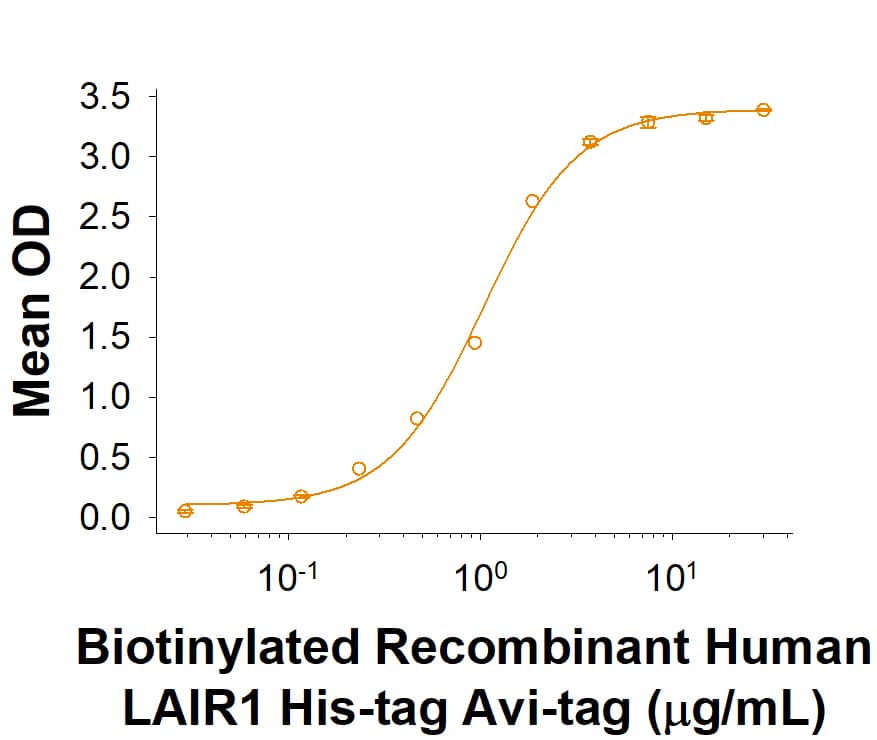
When Bovine Collagen I is coated at 10 µg/mL, 100 μL/well, Biotinylated Recombinant Human LAIR1 His-tag Avi-tag (Catalog # AVI2664) binds with an ED50 of 0.6-4.8 μg/mL.
Scientific Data Images for Recombinant Human LAIR1 His-tag Avi-tag Protein, CF
Recombinant Human LAIR1 His-tag Avi-tag Protein Binding Activity
When Bovine Collagen I is coated at 10 μg/mL, 100 μL/well, Biotinylated Recombinant Human LAIR1 His-tag Avi-tag (Catalog # AVI2664) binds with an ED50 of 0.6-4.8 μg/mL.Recombinant Human LAIR1 His-tag Avi-tag Protein SDS-PAGE
2 μg/lane of Recombinant Human LAIR1 His-tag Avi-tag (Catalog # AVI2664) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 25-38 kDa.Formulation, Preparation and Storage
AVI2664
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: LAIR1
LAIR1 (leukocyte-associated Ig-like receptor-1, designated CD305) is an approximately 40 kDa type I transmembrane inhibitory glycoprotein belonging to the Ig superfamily (1-4). LAIR1 is a collagen-binding protein that is expressed in a differentiation- and activation-dependent manner on most immune cells, including T, B, NK and dendritic cells (DC), monocytes, CD34+ hematopoietic progenitors, most thymocytes, and selected granulocyte populations (2-7). Mature human LAIR1 is a 266 amino acid (aa) type I transmembrane protein that includes a 144 aa extracellular domain (ECD) with one collagen-binding C2-type Ig-like domain, and a 101 aa cytoplasmic domain with two ITIM motifs (2, 3, 8, 9). Of four potential human LAIR1 splice variants, LAIR1b has a 17 aa deletion within the ECD, but outside the Ig domain. LAIR1c differs from LAIR1b by one aa. LAIR1d has a 78 aa cytoplasmic truncation and lacks ITIM motifs. Human LAIR1 ECD shares <45% aa sequence identity with mouse, rat, bovine or canine LAIR1 ECD, but all are functional orthologs. Humans, but not rodents, also express the 152 aa secreted protein LAIR2, which shares 83% aa sequence identity with the LAIR1 ECD up to aa 140 and can block LAIR1 collagen binding (1, 2). A soluble form of LAIR1 found in plasma and urine also binds collagen (10). Adhesion of LAIR1 to collagens in the extracellular matrix, transmembrane collagens expressed by tumor cells, or antibody-mediated crosslinking of LAIR1, inhibits signals relayed by ITAM-bearing receptors and some cytokine-mediated signals (6-8, 13). Processes that are inhibited include B and T cell receptor-mediated activation, NK and T cell‑mediated cytotoxicity, and basophil degranulation (1-4, 8). LAIR1 is reduced or absent on chronic lymphocytic leukemia (CLL) B cells, and some B and DC cells in systemic lupus erythematosus (SLE). Its under‑expression potentially enhances CLL proliferation and SLE immune responses (7, 11, 12).
References
- Meyaard, L. (2008) J. Leukoc. Biol. 83:799.
- Meyaard, L. et al. (1997) Immunity 7:283.
- Lebbink, R.J. et al. (2004) J. Immunol. 172:5535.
- Ouyang, W. et al. (2003) Biochem. Biophys. Res. Commun. 310:1236.
- Verbrugge, A. et al. (2006) J. Leukoc. Biol. 79:828.
- Lebbink, R.J. et al. (2006) J. Exp. Med. 203:1419.
- Bonaccorsi, I. et al. (2010) PLoS ONE 5:e15080.
- Tang, X. et al. (2009) J. Immunol. 182:5446.
- Brondijk, T.H.C. et al. (2010) Blood 115:1364.
- Olde Nordkamp, M.J. et al. (2011) Arthritis Rheum. 63:3749.
- Poggi, A. et al. (2008) Leukemia 22:980.
- Colombo, B.M. et al. (2012) PLoS ONE 7:e31903.
- Rygiel, T.P. et al. (2011) Mol. Immunol. 49(1-2):402.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional LAIR1 Products
Product Documents for Recombinant Human LAIR1 His-tag Avi-tag Protein, CF
Product Specific Notices for Recombinant Human LAIR1 His-tag Avi-tag Protein, CF
For research use only