Recombinant Human Fc gamma RIIIA Alexa Fluor® 488 Protein
R&D Systems, part of Bio-Techne | Catalog # AFG4325

Key Product Details
- R&D Systems NS0-derived Recombinant Human Fc gamma RIIIA Alexa Fluor® 488 Protein (AFG4325)
- Quality control testing to verify active proteins with lot specific assays by in-house scientists
- All R&D Systems proteins are covered with a 100% guarantee
Source
Accession #
Structure / Form
Excitation Wavelength: 488 nm
Emission Wavelength: 515–545 nm
Conjugate
Applications
Product Specifications
Source
Gly17-Gln208, with a C-terminal 6-His tag
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
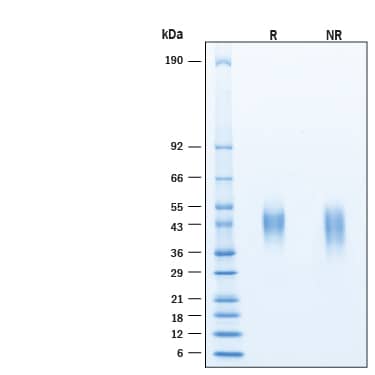
SDS-PAGE
Activity
Scientific Data Images for Recombinant Human Fc gamma RIIIA Alexa Fluor® 488 Protein
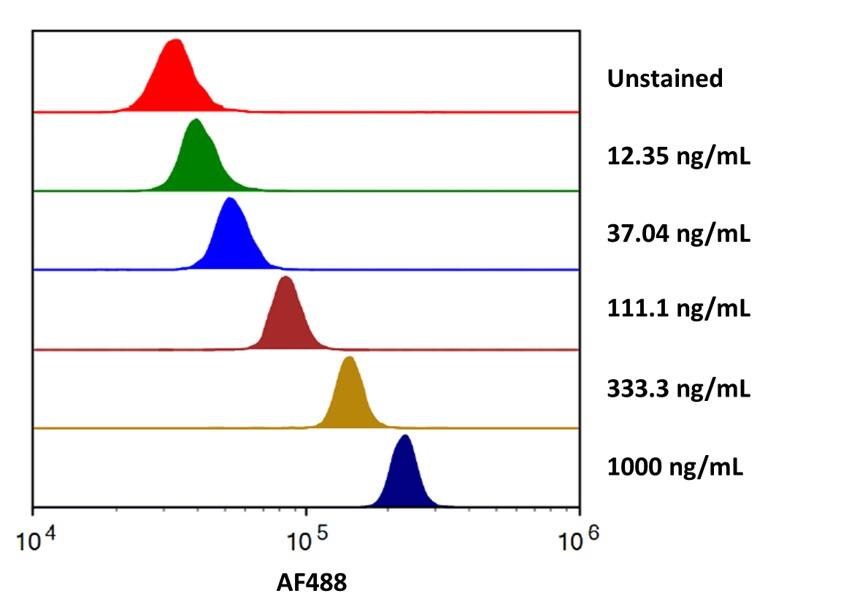
Flow cytometry analysis for Recombinant Human Fc gamma RIIIA/CD16a Alexa Fluor® 488 staining on Human Fc gamma RIIIA/CD16a Antibody conjugated beads.
Streptavidin coated beads conjugated to Human Fc gamma RIII (CD16) Biotinylated Antibody (FAB2546B) were stained with the indicated concentrations of Recombinant Human Fc gamma RIIIA/CD16a Alexa Fluor® 488 (Catalog # AFG4325).Recombinant Human Fc gamma RIIIA/CD16a Alexa Fluor® 488 Protein SDS-PAGE.
2 μg/lane of Recombinant Human Fc gamma RIIIA/CD16a Alexa Fluor® 488 Protein (Catalog # AFG4325) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 40-50 kDa.Formulation, Preparation and Storage
AFG4325
| Formulation | Supplied as a 0.2 μm filtered solution in PBS with BSA as a carrier protein. |
| Shipping | The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Protect from light. Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Fc gamma RIIIA/CD16a
Fc gamma RIIIa is a low/intermediate affinity receptor for polyvalent immune-complexed IgG. It is involved in phagocytosis, secretion of enzymes and inflammatory mediators, antibody-dependent cytotoxicity and clearance of immune complexes (1, 2). In humans, it is a 50-70 kDa type I transmembrane activating receptor expressed by NK cells, T cells, monocytes, and macrophages (1). Fc gamma RIIIb is highly related, sharing 97% amino acid (aa) identity within the extracellular domain (ECD), but is a GPI-linked receptor expressed on human neutrophils and eosinophils (1, 2). The ECD of Fc gamma RIIIa shares 63%, 61%, 65%, 59% and 58% aa identity with mouse Fc gamma RIV, rat Fc gamma RIIIa, feline CD16, bovine CD16 and porcine Fc gamma RIIIb paralogs, respectively. The Fc gamma RIIIa cDNA encodes 254 aa including a 16 aa signal sequence, 191 aa ECD with two C2-type Ig-like domains and five potential N-glycosylation sites, a 22 aa transmembrane (TM) sequence and a 25 aa cytoplasmic domain. In humans, a single nucleotide polymorphism creates high binding (176V) and low binding (176F) forms that, when homozygous, may influence susceptibility to autoimmune diseases or response to therapeutic IgG antibodies (3, 4). Catalog # 4325-FC is expressed as the 176V isoform of Fc gamma RIIIa. Fc gamma RIIIa surface expression requires interaction of an accessory chain, either the common gamma-chain or CD3 zeta (5, 6). Glycosylation patterns, electrophoretic mobility and binding affinity appear to differ between NK cell and monocyte Fc gamma RIIIa (7). The ECD of both Fc gamma RIIIa and b can be proteolytically cleaved and retain binding activity in soluble form (8-11). In monocytes and macrophages, activation and phagocytosis can trigger Fc gamma RIIIa release (11). Soluble Fc gamma RIII can be detected in normal plasma and is increased in rheumatoid arthritis and in coronary artery diseases (9, 10).
References
- Nimmerjahn, F. and J.V. Ravetch (2006) Immunity 24:19.
- Ravetch, J.V. and B. Perussia (1989) J. Exp. Med. 170:481.
- Wu, J. et al. (1997) J. Clin. Invest. 100:1059.
- Dall’Ozzo, S. et al. (2004) Cancer Res. 64:4664.
- Kim, M.-K. et al. (2003) Blood 101:4479.
- Lanier, L.L. et al. (1989) Nature 342:803.
- Edberg, J.C. and R.P. Kimberley (1997) J. Immunol. 159:3849.
- Li, P. et al. (2007) J. Biol. Chem. 282:6210.
- Masuda, M. et al. (2003) J. Rheumatol. 30:1911.
- Masuda, M. et al. (2006) Atherosclerosis 188:377.
- Webster, N.L. et al. (2006) J. Leukoc. Biol. 79:294.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional Fc gamma RIIIA/CD16a Products
Product Documents for Recombinant Human Fc gamma RIIIA Alexa Fluor® 488 Protein
Product Specific Notices for Recombinant Human Fc gamma RIIIA Alexa Fluor® 488 Protein
This product is
provided under an agreement between Life Technologies Corporation and R&D
Systems, Inc, and the manufacture, use, sale or import of this product is
subject to one or more US patents and corresponding non-US equivalents, owned
by Life Technologies Corporation and its affiliates. The purchase of this
product conveys to the buyer the non-transferable right to use the purchased
amount of the product and components of the product only in research conducted
by the buyer (whether the buyer is an academic or for-profit entity). The sale
of this product is expressly conditioned on the buyer not using the product or
its components (1) in manufacturing; (2) to provide a service, information, or
data to an unaffiliated third party for payment; (3) for therapeutic,
diagnostic or prophylactic purposes; (4) to resell, sell, or otherwise transfer
this product or its components to any third party, or for any other commercial
purpose. Life Technologies Corporation will not assert a claim against the
buyer of the infringement of the above patents based on the manufacture, use or
sale of a commercial product developed in research by the buyer in which this
product or its components was employed, provided that neither this product nor
any of its components was used in the manufacture of such product. For
information on purchasing a license to this product for purposes other than
research, contact Life Technologies Corporation, Cell Analysis Business Unit,
Business Development, 29851 Willow Creek Road, Eugene, OR 97402, Tel: (541)
465-8300. Fax: (541) 335-0354.
This product is provided under an agreement between Life Technologies Corporation and R&D Systems, Inc, and the manufacture, use, sale or import of this product is subject to one or more US patents and corresponding non-US equivalents, owned by Life Technologies Corporation and its affiliates. The purchase of this product conveys to the buyer the non-transferable right to use the purchased amount of the product and components of the product only in research conducted by the buyer (whether the buyer is an academic or for-profit entity). The sale of this product is expressly conditioned on the buyer not using the product or its components (1) in manufacturing; (2) to provide a service, information, or data to an unaffiliated third party for payment; (3) for therapeutic, diagnostic or prophylactic purposes; (4) to resell, sell, or otherwise transfer this product or its components to any third party, or for any other commercial purpose. Life Technologies Corporation will not assert a claim against the buyer of the infringement of the above patents based on the manufacture, use or sale of a commercial product developed in research by the buyer in which this product or its components was employed, provided that neither this product nor any of its components was used in the manufacture of such product. For information on purchasing a license to this product for purposes other than research, contact Life Technologies Corporation, Cell Analysis Business Unit, Business Development, 29851 Willow Creek Road, Eugene, OR 97402, Tel: (541) 465-8300. Fax: (541) 335-0354.
For research use only