Recombinant H. pylori Fucosyltransferase Protein, CF
R&D Systems, part of Bio-Techne | Catalog # 11072-GT

Key Product Details
Product Specifications
Source
Met1-Tyr392, with a C-terminal 6-His tag
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
SDS-PAGE
Activity
The specific activity is >1500 pmol/min/μg, as measured under the described conditions.
Scientific Data Images for Recombinant H. pylori Fucosyltransferase Protein, CF
Recombinant H. pylori Fucosyltransferase Protein Enzyme Activity Diagram.
Recombinant H. pylori Fucosyltransferase His-tag Protein (Catalog # 11072-GT) Enzyme Activity Diagram.Fluorescent glycan labeling of asialofetuin (AF) with Cy3-Fuc using rHp. FUT.
Asialofetuin (4 μg) was incubated with 0.2 nmol of GDP-Cy3-Fucose (ES401) with or without rHp. FUT (Catalog # 11072) (1.0 μg) in 25 μL of 25 mM Tris pH 7.5, 10 mM MnCl2 at 37 °C for 30 minutes. Following labeling, half of the AF was deglycosylated with PNGase F N-glycan Releasing Kit (EA006) and resolved in 15% SDS-PAGE. Left side is the TCE image and right side is the Fluorescent image of the same gel.Recombinant H. pylori Fucosyltransferase Protein SDS-PAGE.
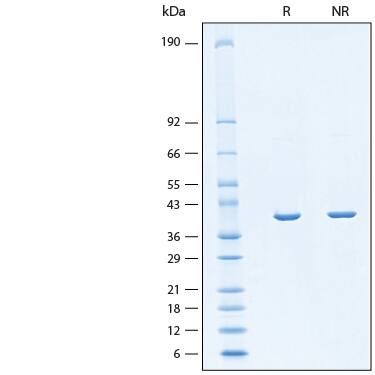
2 μg/lane of Recombinant H. pylori Fucosyltransferase Protein (Catalog # 11072-GT) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 39 kDa.Formulation, Preparation and Storage
11072-GT
| Formulation | Supplied as a 0.2 μm filtered solution in Tris and NaCl. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Fucosyltransferase
Lewis X (LeX), a fucosylated trisaccharide glycan epitope (Gal beta1,4 [Fuc alpha1,3]GlcNAc beta) also known as CD15, and sialylated Lewis X (sLeX) are distributed throughout eukaryotes and are determinants of many functional glycoconjugates that play central roles in numerous physiological and pathological processes (1, 2). Lex-bearing glycans are also found in the infectious bacterium Helicobacter pylori to mask the bacterium from the host immune surveillance (3). H. pylori is the pathogen that causes peptic ulcers that can further lead to stomach cancer and gastritis. H. pylori fucosyltransferase is responsible for generating the LeX glycans, therefore is potentially a drug target for curing peptic ulcers, gastritis and stomach cancer. In molecular terms, H. pylori fucosyltransferase is an alpha1,3 fucosyltransferase that shares function with human FUT4, FUT5, FUT6, and FUT9 (4). Remarkably, H. pylori fucosyltransferase can transfer IgG antibody to the glycocalyx on the surfaces of live cells when the antibody is conjugated to the enzyme's natural donor substrate GDP-Fucose (5). The activity of this enzyme has been measured with a phosphatase coupled method (6).
References
- Gooi, H.C. et al. (1981) Nature 292:156.
- Phillips, M.L. et al. (1990) Science 250:1130.
- Moran, A.P. et al. (1996) FEMS Immunol Med Microbiol 16:105.
- de Vries, T. et al. (2001). Glycobiology 11:119R.
- Li, J. et al. (2018) ACS Cent. Sci. 4:1633.
- Wu, Z.L. et al. (2011) Glycobiology 21:727.
Long Name
Alternate Names
UniProt
Additional Fucosyltransferase Products
Product Documents for Recombinant H. pylori Fucosyltransferase Protein, CF
Product Specific Notices for Recombinant H. pylori Fucosyltransferase Protein, CF
For research use only