Mouse Galectin-1 PE-conjugated Antibody
R&D Systems, part of Bio-Techne | Catalog # IC1245P

Key Product Details
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Ala2-Glu135
Accession # P16045
Specificity
Clonality
Host
Isotype
Scientific Data Images for Mouse Galectin-1 PE-conjugated Antibody
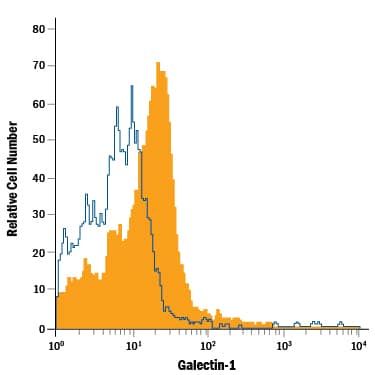
Detection of Galectin‑1 in Mouse Splenocytes by Flow Cytometry.
Mouse splenocytes were stained with Goat Anti-Mouse Galectin-1 PE-conjugated Antigen Affinity-purified Polyclonal Antibody (Catalog # IC1245P, filled histogram) or isotype control antibody (Catalog # IC108P, open histogram). To facilitate intracellular staining, cells were fixed with Flow Cytometry Fixation Buffer (Catalog # FC004) and permeabilized with Flow Cytometry Permeabilization/Wash Buffer I (Catalog # FC005). View our protocol for Staining Intracellular Molecules.Applications for Mouse Galectin-1 PE-conjugated Antibody
Intracellular Staining by Flow Cytometry
Sample: Mouse splenocytes fixed with Flow Cytometry Fixation Buffer (Catalog # FC004) and permeabilized with Flow Cytometry Permeabilization/Wash Buffer I (Catalog # FC005)
Formulation, Preparation, and Storage
Purification
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, 2 to 8 °C as supplied.
Background: Galectin-1
The galectins constitute a large family of carbohydrate-binding proteins with specificity for N-acetyl-lactosamine-containing glycoproteins. At least 14 mammalian galectins, which share structural similarities in their carbohydrate recognition domains (CRD), have been identified to date. Galectin-1 has been classified as a prototype galectin (-1, -2, -5, -7, -10, -11, -13, -14), which contains one CRD and exists either as a monomer or a noncovalent homodimer. The chimera galectins (such as Galectin-3) contain one CRD linked to a nonlectin domain, while the tandem-repeat galectins (-4, -6, -8, -9, -12) consist of two CRDs joined by a linker peptide. Galectins lack a classical signal peptide and can be localized to the cytosolic compartments where they have intracellular functions. However, via one or more as yet unidentified non-classical secretory pathways, galectins can also be secreted and function extracellularly. Individual members of the galectin family have different tissue distribution profiles and exhibit subtle differences in their carbohydrate-binding specificities. Each family member may preferentially bind to a unique subset of cell-surface glycoproteins (1-5).
Mouse Galectin-1, also known as beta-galactoside-binding lectin L-14-I, lactose-binding lectin 1, S-Lac lectin 1, galaptin and 14 kDa lectin, is a monomeric or homodimeric prototype galectin that is expressed in a variety of tissues and cells including muscle, heart, lymph nodes, spleen, thymus, macrophages, B cells, T cells, dendritic cells, and tumor cells. It preferentially binds laminin, fibronectin, 90K/Mac-2BP, CD45, CD43, CD7, CD2, CD3, and ganglioside GM1. Galectin-1 modulates cell growth, proliferation and differentiation, either positively or negatively, depending on the cell type and activation status. It controls cell survival by inducing apoptosis of activated T cells and immature thymocytes. It modulates cytokine secretion by inducing Th2 type cytokines and inhibiting pro-inflammatory cytokine production. Galectin-1 can also modulate cell-cell as well as cell-matrix interactions, and depending on the cell type and developmental stage, promote cell attachment or detachment. Galectin-1 has immunosuppressive and anti-inflammatory properties, and has been shown to suppress acute and chronic inflammation and autoimmunity. Mouse and human Galectin-1 share about 88% amino acid sequence similarity (1-6).
References
- Rabinovich, A. et al. (2002) Trends Immunol. 23:313.
- Rabinovich, A. et al. (2002) J. Leukoc. Biol. 71:741.
- Hughes, R.C. (2001) Biochimie 83:667.
- Viquier, M. et al. (2014) Tissue Barriers 2:e29103.
- Compagno, D. et al. (2014) Curr. Mol. Med. 14:630.
- Goldring, K. et al. (2002) J. Cell Sci. 115:355.
Alternate Names
Gene Symbol
UniProt
Additional Galectin-1 Products
Product Documents for Mouse Galectin-1 PE-conjugated Antibody
Product Specific Notices for Mouse Galectin-1 PE-conjugated Antibody
For research use only