Anti- Solanezumab (Anti-Idiotype) Antibody
R&D Systems, part of Bio-Techne | Catalog # MAB10124
Recombinant Monoclonal Antibody.

Conjugate
Catalog #
Key Product Details
Species Reactivity
Multi-Species
Applications
CyTOF-ready, Flow Cytometry, Western Blot
Label
Unconjugated
Antibody Source
Recombinant Monoclonal Rabbit IgG Clone # 2372A
Product Specifications
Immunogen
Full-length Solanezumab
Specificity
Detects Solanezumab in Western blots and direct ELISAs. In Western blots, no cross-reactivity with Trastuzumab and Adalimumab is observed.
Clonality
Monoclonal
Host
Rabbit
Isotype
IgG
Scientific Data Images for Anti- Solanezumab (Anti-Idiotype) Antibody
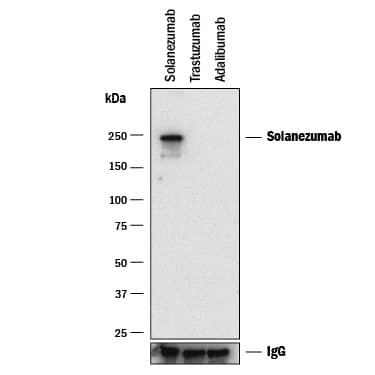
Detection of Solanezumab by Western Blot.
Western blot shows Solanexumab, Trastuzumab (negative control), and Adalibumab (negative control). PVDF membrane was probed with 1 µg/mL of Rabbit Anti-Anti- Solanezumab (Anti-Idiotype) Monoclonal Antibody (Catalog # MAB10124) followed by HRP-conjugated Anti-Rabbit IgG Secondary Antibody (Catalog # HAF008). A specific band was detected for Solanezumab at approximately 240 kDa (as indicated). Human IgG is shown as a loading control. This experiment was conducted under non-reducing conditions and using Immunoblot Buffer Group 1.Detection of Anti-APP (Solanezumab) on Human PBMC by flow cytometry.
Human PBMC were stained with Human anti-Human APP (Solanezumab) Monoclonal Antibody (Catalog # MAB9919), then either (A) Rabbit Anti-Solanezumab (Anti-Idiotype) (Catalog # MAB10124) or (B) normal rabbit IgG (Catalog # MAB1050) followed by APC-conjugated anti-rabbit IgG secondary antibody (Catalog # F0111) and Mouse anti-Human CD14 PE-conjugated Monoclonal Antibody (Catalog # FAB3832P).Applications for Anti- Solanezumab (Anti-Idiotype) Antibody
Application
Recommended Usage
CyTOF-ready
Ready to be labeled using established conjugation methods. No BSA or other carrier proteins that could interfere with conjugation.
Flow Cytometry
0.25 µg/106 cells
Sample: Human PBMCs stained with Human anti-Human APP (Solanezumab), Catalog # MAB9919
Sample: Human PBMCs stained with Human anti-Human APP (Solanezumab), Catalog # MAB9919
Western Blot
1 µg/mL
Sample: Solanezumab
Under non-reducing conditions only
Sample: Solanezumab
Under non-reducing conditions only
Formulation, Preparation, and Storage
Purification
Protein A or G purified from cell culture supernatant
Reconstitution
Reconstitute at 0.5 mg/mL in sterile PBS. For liquid material, refer to CoA for concentration.
Formulation
Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. *Small pack size (SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Shipping
Lyophilized product is shipped at ambient temperature. Liquid small pack size (-SP) is shipped with polar packs. Upon receipt, store immediately at the temperature recommended below.
Stability & Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Solanezumab
Additional Solanezumab Products
Product Documents for Anti- Solanezumab (Anti-Idiotype) Antibody
Product Specific Notices for Anti- Solanezumab (Anti-Idiotype) Antibody
For research use only
Loading...
Loading...
Loading...
Loading...
Loading...
Loading...
