Recombinant Mouse PDGF-AB Protein, CF
R&D Systems, part of Bio-Techne | Catalog # 10839-AB

Key Product Details
- R&D Systems E. coli-derived Recombinant Mouse PDGF-AB Protein (10839-AB)
- Quality control testing to verify active proteins with lot specific assays by in-house scientists
- All R&D Systems proteins are covered with a 100% guarantee
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
Source
| Mouse PDGF-A (Ser87-Thr211) Accession # P20033.2 |
|
| Mouse PDGF-B (Ser82-Thr190) Accession # P31240.1 |
|
| N-terminus | C-terminus |
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
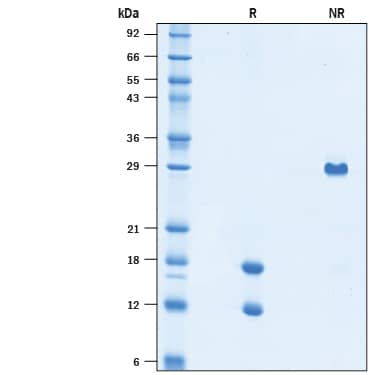
SDS-PAGE
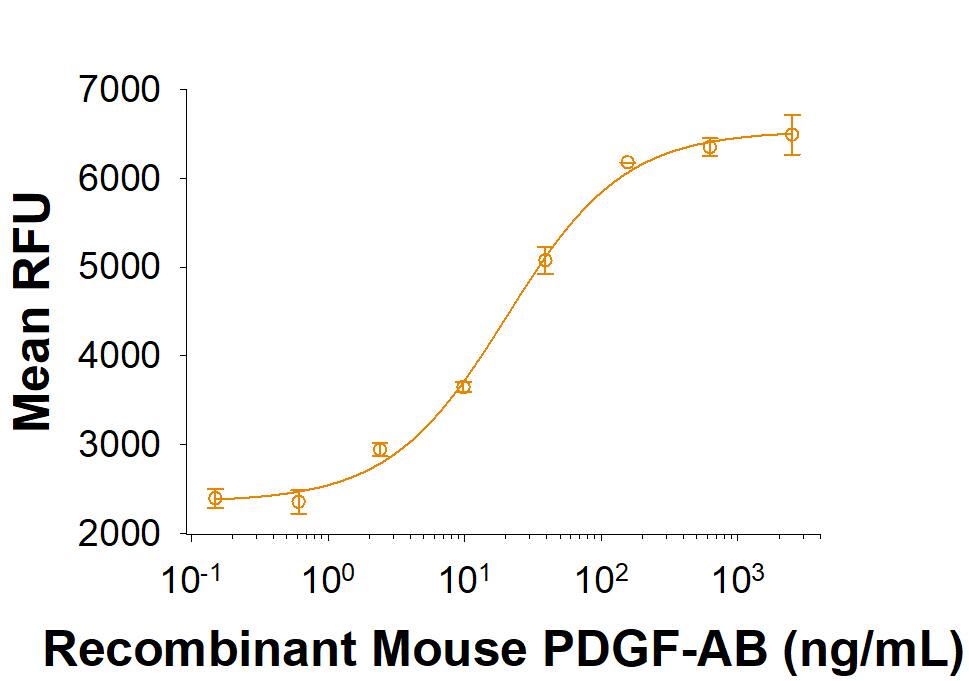
Activity
The ED50 for this effect is 15.0-75.0 ng/mL.
Scientific Data Images for Recombinant Mouse PDGF-AB Protein, CF
Recombinant Mouse PDGF-AB Protein Bioactivity.
Recombinant Mouse PDGF-AB induces NR6R-3T3 mouse fibroblast cell proliferation. The ED50 for this effect is 15.0-75.0 ng/mL.Recombinant Mouse PDGF-AB Protein SDS-PAGE.
2 µg/lane of Recombinant Mouse PDGF-AB (Catalog # 10839-AB) was resolved by SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 17.5 kDa (A chain) & 12.5 kDa (B chain) and 29 kDa (heterodimer), respectively.Formulation, Preparation and Storage
10839-AB
| Formulation | Lyophilized from a 0.2 μm filtered solution in Acetonitrile and TFA. |
| Reconstitution | Reconstitute at 100 μg/mL in 4 mM HCl. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: PDGF-AB
Platelet-Derived Growth Factors (PDGFs) are a family of four cystine-knot-type growth factors (PDGF-A, -B, -C and -D) which control the growth of connective tissue cells such as fibroblasts and smooth muscle cells (1, 2). They comprise of four homodimers (PDGF-AA, PDGF-BB, PDGF-CC and PDGF-DD) and one heterodimer (PDGF-AB). All four PDGF genes contain a signal sequence, a propeptide and a mature form (2, 3). Mature mouse PDGFA is 95% and 98% identical to human and rat PDGFA respectively whereas mature mouse PDGFB is 89% and 98% homologous to human and rat PDGFB respectively. In general, PDGFs are expressed by a number of different cells and tissues including endothelial cells, epithelial cells, hematopoietic cells, connective tissue, nervous tissue, brain, muscle, kidney and liver. PDGF expression is highly regulated with PDGF levels increasing following injury and/or disease. An analysis of PDGF purified from human platelets suggest that approximately 70% of sera-derived PDGF is PDGF-AB (4). PDGF isoforms exert their cellular effect by binding to the structurally similar receptor tyrosine kinases PDGF receptor-alpha and PDGF receptor-beta. PDGF-AB binding induces receptor dimerization resulting in alpha alpha receptor homodimers and alpha beta receptor heterodimers. Binding of PDGF receptors has been reported to result in stimulation of mitogenicity and chemotaxis of fibroblasts, stimulation of granule release by neutrophils and monocytes, facilitation of steroid synthesis by Leydig cells, stimulation of neutrophil phagocytosis, stimulation of collagen, fibronectin, proteoglycan, and hyaluronic acid synthesis, modulation of thrombospondin expression and secretion, stimulation of collagenase activity and secretion, induction of contraction of rat aorta strips in vitro, and transient induction of T cell IL-2 secretion accompanied by a down-regulation of IL-4 and IFN-gamma production (5).
References
- Kohler, N. Lipton, A. (1974) Exp Cell Res. 87:297.
- Ross, R. et al. (1974) Proc Natl Acad Sci U.S.A. 71:1207.
- Frediksson, L. et al. (2004) Cytokine Growth Factor Rev. 15:197.
- Chen, P-H. et al. (2013) BiochimBiophysActa 1834:2176.
- Heldin, C-H. Eriksson, U. and Ostman, A. (2002) Arch. Biochem. Biophysic. 398:284.
Long Name
Alternate Names
Additional PDGF-AB Products
Product Documents for Recombinant Mouse PDGF-AB Protein, CF
Product Specific Notices for Recombinant Mouse PDGF-AB Protein, CF
For research use only