Recombinant Human PILR-alpha Avi-tag His-tag Protein, CF
R&D Systems, part of Bio-Techne | Catalog # AVI6484

Key Product Details
- R&D Systems CHO-derived Recombinant Human PILR-alpha Avi-tag His-tag Protein (AVI6484)
- Quality control testing to verify active proteins with lot specific assays by in-house scientists
- All R&D Systems proteins are covered with a 100% guarantee
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
Source
| Human PILR-alpha (Thr25-Thr196) Accession # Q9UKJ1.3 |
Avi-tag | 6-His tag |
| N-terminus | C-terminus |
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
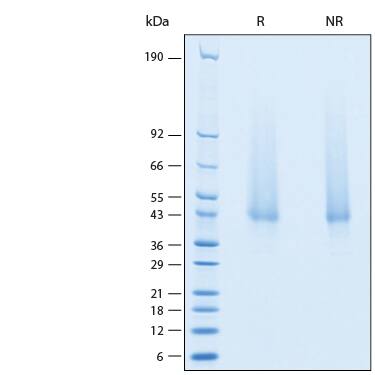
SDS-PAGE
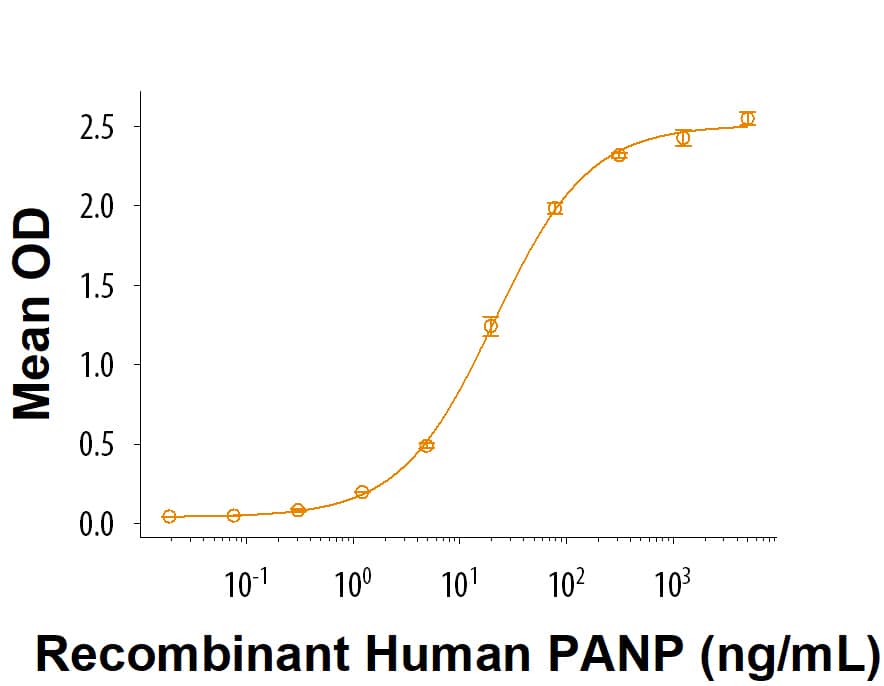
Activity
When Biotinylated Recombinant Human PILR‑ alpha Avi-tag His-tag (Catalog # AVI6484) is captured on EvenCoat Streptavidin Coated Plates (Catalog # CP004) at 2.00 μg/mL (100 μL/well), Recombinant Human PANP Fc Chimera Protein (Catalog # 7920-PN) binds with an ED50 of 8.00-80.0 ng/mL.
Scientific Data Images for Recombinant Human PILR-alpha Avi-tag His-tag Protein, CF
Recombinant Human PILR-alpha Avi-tag His-tag Protein Binding Activity.
Measured by its binding ability in a functional ELISA. When Biotinylated Recombinant Human PILR‑alpha Avi-tag His-tag (Catalog # AVI6484) is captured on EvenCoat Streptavidin Coated Plates (CP004) at 2.00 μg/mL (100 μL/well), Recombinant Human PANP Fc Chimera Protein (7920-PN) binds with an ED50 of 8.00-80.0 ng/mL.Recombinant Human PILR-alpha Avi-tag His-tag Protein SDS-PAGE.
2 μg/lane of Recombinant Human PILR-alpha Avi-tag His-tag Protein (Catalog # AVI6484) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 39-47 kDa.Formulation, Preparation and Storage
AVI6484
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: PILR-alpha
PILR-alpha (paired immunoglobulin-like type 2 receptor-alpha; also FDF03) is one of two members that belong to a small family of immunoregulatory Ig-superfamily receptors (1-4). It is a counterpart to PILR-beta, and likely gave rise to the PILR-beta gene through duplication and rearrangement (1). The PILRs represent one of many pairs of Ig-like domain-containing receptors that participate in immune regulation. PILR-alpha and -beta should not be confused with the similarly named PIRs (also paired immunoglobulin-like receptors ), or the functionally-related SIRP and ILT/LILR/CD85/LIR family of receptors (2). While PIRs, ILTs and SIRPs contain three to six Ig‑like domains in their extracellular region, PILR-alpha and -beta show only one Ig-like region in their extracellular domain (ECD) (1-5). Human PILR-alpha is a monomeric, 55 kDa, 294 amino acid (aa) type I transmembrane (TM) glycoprotein (3-5). It contains a 178 aa ECD (aa 20-197), a 21 aa TM segment, and a long, 85 aa cytoplasmic region (aa 219-303). The ECD shows one V-type Ig-like domain between aa 32-150, while the cytoplasmic region contains two ITIMs (immunoreceptor Tyr-based inhibitory motifs) between aa 267-272 and 296-301. Given that ITIMs are known to interact with phosphatases such as PTPN6 and PTPN11, the presence of these motifs make mouse PILR-alpha an inhibitory receptor. Three potential isoforms for human PILR-alpha have been reported. The first contains a 24 aa substitution for aa 152‑303, a second possesses a 36 aa substitution for aa 264-303, and a third shows a deletion of aa 152-224 (6). The human PILR-alpha ECD shares 43% aa sequence identity with mouse PILR-alpha ECD, and 82% aa sequence identity with the ECD of human PILR-beta (3, 4).
PILR-alpha is expressed by neutrophils, macrophages, monocytes, mast cells, APCs, microglia, neurons, cardiac muscle and renal proximal plus pancreatic duct eipthelium (4, 7, 8). It has multiple binding partners, including CD99 (4, 9), glycoprotein B/gB of HSV-1 (7), PANP (PILR-associated neural protein) (8) and NPDC1 plus collectin-12 (10). Although PILR-alpha and -beta are related through gene duplication and highly similar in their ECD aa sequence, they do not necessarily share the same ligands (or binding partners), as PILR-beta fails to bind to gB and PANP (8, 10). Notably, PILR-alpha binding appears to be dependent upon the presence of a poorly-defined peptide sequence coupled to a sialylated, O-linked carbohydrate motif (5, 9-12). It is unclear what function(s) can be attributed to PILR-alpha. One possibility suggests that in the early stage of an immune response, PILR-beta predominates over PILR-alpha on the APC surface. Ligation of PILR-beta by CD99 induces IL-12 production and immune cell activation. But this ligation also up‑regulates PILR-alpha expression, and subsequent CD99:PILR‑ alpha engagement now promotes IL-27 production, with a concomitant increase in T cell IL-10 production, and a down‑regulation of the inflammatory response (10). Our Avi-tag Biotinylated PILR-alpha protein features biotinylation at a single site contained within the Avi-tag, a unique 15 amino acid peptide. Protein orientation will be uniform when bound to streptavidin-coated surface due to the precise control of biotinylation and the rest of the protein is unchanged so there is no interference in the protein's bioactivity.
References
- Wilson, M.D. et al. (2006) Physiol. Genomics 27:201.
- Lanier, L.L. (2001) Curr. Opin. Immunol. 13:326.
- Fournier, N. et al. (2000) J. Immunol. 165:1197.
- Shiratori, I. et al. (2004) J. Exp. Med. 199:525.
- Mousseau, D.D. et al. (2000) J. Biol. Chem. 275:4467.
- SwissProt Accession # Q9UKJ1.
- Tato, C.M. et al. (2012) PLoS ONE 7:e31680.
- Satoh, T. et al. (2008) Cell 132:935.
- Tabata, S. et al. (2008) J. Biol. Chem. 283:8893.
- Sun, Y. et al. (2012) J. Biol. Chem. 287:15837.
- Wang, J. et al. (2008) J. Biol. Chem. 180:1686.
- Arii, J. et al. (2010) J. Virol. 84:10733.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional PILR-alpha Products
Product Documents for Recombinant Human PILR-alpha Avi-tag His-tag Protein, CF
Product Specific Notices for Recombinant Human PILR-alpha Avi-tag His-tag Protein, CF
For research use only