Recombinant Human MFAP3L Fc Chimera Protein, CF
R&D Systems, part of Bio-Techne | Catalog # 10798-MF

Key Product Details
- R&D Systems HEK293-derived Recombinant Human MFAP3L Fc Chimera Protein (10798-MF)
- Quality control testing to verify active proteins with lot specific assays by in-house scientists
- All R&D Systems proteins are covered with a 100% guarantee
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
Source
| Human MFAP3L (Lys29-Met149) Accession # O75121.3 |
IEGRMD | Human IgG1 (Pro100-Lys330) |
| N-terminus | C-terminus |
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
SDS-PAGE
Activity
Scientific Data Images for Recombinant Human MFAP3L Fc Chimera Protein, CF
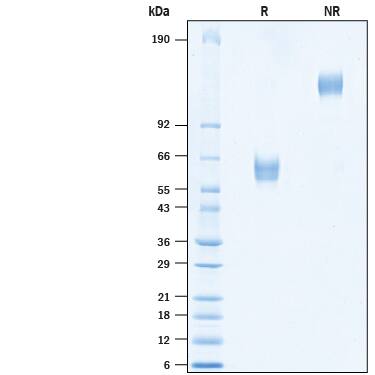
Recombinant Human MFAP3L Fc Chimera Protein SDS-PAGE.
2 μg/lane of Recombinant Human MFAP3L Fc Chimera (Catalog # 10798-MF) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 54-66 kDa and 108-132 kDa, respectively.Formulation, Preparation and Storage
10798-MF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution | Reconstitute at 500 µg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: MFAP3L
Microbibrillar-Associated Protein 3-Like (MFAP3L), also known as NYD-sp9, is part of the microfibrillar-associated protein family (MFAPs). MFAPs are non‐fibrillin, extracellular matrix glycoproteins that interact with fibrillin and were originally characterized in microfibrillar assembly (1,2). In humans, there several subfamily members with varying amino acid (aa) sequence homology and functions (1,2). Among the family, MFAP2 and MFAP5 are more closely related and while MFAP1, 3 and 4 share no structural or sequence homology with MFAP2, MFAP5 or with each other (1,2). Human MFAP3L shows 71% amino acid (aa) sequence homology to MFAP3, but not other MFAPs (3). Mature, human MFAP3L consists of an extracellular domain (ECD) containing N-linked glycosylation sites, a transmembrane domain, and a cytoplasmic domain with a conserved SH2 motif (3). The ECD of human MFAP3L shares 89% and 90% aa sequence identity with mouse and rat MFAP3L, respectively. MFAPs have the unique ability to interact with TGF-beta family growth factors, Notch and Notch ligands and multiple elastic fiber proteins, in addition to interacting with fibrillin (1, 2). MFAPs are expressed in a wide variety of tissues and, along with microfibril assembly, they play roles in the regulation of tissue homeostasis, cell survival, and tumor progression (1,2). MFAP3L is often located within colorectal cancer (CRC) cells, which metastasize by activation of the nuclear ERK pathway via MFAP3L phosphorylation (3). Regulation of this MFAP3L activity could have pharmaceutical effects on CRC tumor progression (3).
References
- Zhu, S. et al. (2020) J Cell Physio. 236:41.
- Mecham, R.P. et al. (2015) Matrix Biol. 47:13.
- Lou, X. et al. (2014) BBA. 1842:1423.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional MFAP3L Products
Product Documents for Recombinant Human MFAP3L Fc Chimera Protein, CF
Product Specific Notices for Recombinant Human MFAP3L Fc Chimera Protein, CF
For research use only