Recombinant Human LR3 IGF-I/IGF-1 Protein, CF
R&D Systems, part of Bio-Techne | Catalog # 8335-G1

Key Product Details
A synthetic analog of IGF-1 designed specifically to excel in cell culture applications
Features and Benefits- High Purity Is determined by SDS-PAGE (>95%) and reverse phase HPLC (>90%). This is the purest commercially available recombinant human LR3 IGF-1 (Figure 1). The high purity generates excellent performance of the protein.
- Stability Extended half-life in culture provides cost saving and time saving benefits.
- Low Endotoxin R&D Systems' endotoxin specification of <0.01 EU/ug of protein greatly diminishes potential non-specific effects in culture applications.
- Lot-to-Lot Consistency Our robust manufacturing process includes stringent testing and analysis to ensure the same performance across all lots.
- Multigram quantities are available to meet bioproduction demands.
Product Specifications
Source
| MFPAMPLSSLFVN | Human LR3 IGF-I/IGF-1 (Gly49-Ala118 (Glu51Arg)) Accession # P05019 |
| N-terminus | C-terminus |
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
SDS-PAGE
Activity
The ED50 for this effect is 0.3-1.5 ng/mL. IGFBP-3 does not inhibt its activity.
Scientific Data Images for Recombinant Human LR3 IGF-I/IGF-1 Protein, CF
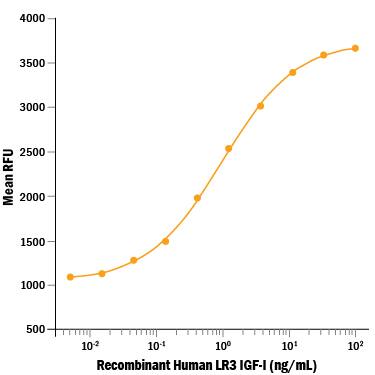
Recombinant Human LR3 IGF-I/IGF-1 Protein Bioactivity
Recombinant Human LR3 IGF-I/IGF-1 (Catalog # 8335-G1) stimulates cell proliferation in a serum-free assay using the MCF-7 human breast cancer cell line. The ED50 for this effect is 0.3-1.5 ng/mL.Recombinant Human LR3 IGF-I/IGF-1 Protein SDS-PAGE
1 μg/lane of Recombinant Human LR3 IGF-I/IGF-1 was resolved with SDS-PAGE under reducing (R) conditions and visualized by silver staining, showing a single band at 7 kDa.Recombinant Human LR3 IGF-I/IGF-1 Protein Mass Spectrometry
ESI analysis of Recombinant Human LR3 IGF-I/IGF-1 (Catalog # 8335-G1). The peak at 9112 Da corresponds to the calculated molecular mass, 9118 Da.Formulation, Preparation and Storage
8335-G1
| Formulation | Lyophilized from a 0.2 μm filtered solution in phosphate buffer, pH 7.2 . |
| Reconstitution | Reconstitute at 500 μg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: IGF-I/IGF-1
Long R3 IGF-I (LR3 IGF-I) is a 9.2 kDa synthetic analog of IGF-I that is generated by modifying the aa sequence for mature human IGF-I. These modifications include the substitution of an Arg for Glu at position 3 of the mature IGF-1 sequence and the addition of a thirteen aa N-terminal extension, which is derived from methionyl porcine Growth Hormone (17). These aa changes generate a protein that is still capable of binding to IGF-I and Insulin receptors, but shows considerably lower affinity binding to IGFBPs compared to wild-type IGF-I (17, 18). As a result, LR3 IGF-I has an increased half-life and displays increased biological potency compared to IGF-I (17-22).
References
- Philippou, A. et al. (2007) In Vivo 21:45.
- Sandberg-Nordqvist, A.C. et al. (1992) Brain Res. Mol. Brain Res. 12:275.
- Berryman, D.E. et al. (2013) Nat. Rev. Endocrinol. 9:346.
- Guvakova, M.A. (2007) Int. J. Biochem. Cell Biol. 39:890.
- Sadagurski, M. and M.F. White (2013) Endocrinol. Metab. Clin. North Am. 42:127.
- Clemmons, D.R. (2006) Curr. Opin. Pharmacol. 6:620.
- Bluher, S. et al. (2005) Best Pract. Res. Clin. Endocrinol. Metab. 19:577.
- Garcia-Segura, L.M. et al. (2006) Neuroendocrinology 84:275.
- Malemud, C.J. (2007) Clin. Chim. Acta 375:10.
- Ling, P.R. et al. (1995) Am. J. Clin. Nutr. 61:116.
- Sheng, M.H. et al. (2014) J. Bone Metab. 21:41.
- Samani, A.A. et al. (2007) Endocrine Rev. 28:20.
- Gallagher, E.J. et al. (2010) Endocr. Pract. 16:864.
- LeRoith, D. and S. Yakar (2007) Nat. Clin. Pract. Endocrinol. Metab. 3:302.
- Denley, A. et al. (2005) Cytokine Growth Factor Rev. 16:421.
- Duan, C. and Q. Xu (2005) Gen. Comp. Endocrinol. 142:44.
- Francis, G.L. et al. (1992) J. Mol. Endocrinol. 8:213.
- Voorhamme, D. and C.A. Yandell (2006) Mol. Biotechnol. 34:201.
- Tomas, F.M. et al. (1993) Biochem. J. 291:781.
- Tomas, F.M. et al. (1996) J. Endocrinol. 150:77.
- Tomas, F.M. et al. (1997) J. Endocrinol. 155:377.
- von der Thüsen, J.H. et al. (2011) Am. J. Pathol. 178:924.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional IGF-I/IGF-1 Products
Product Documents for Recombinant Human LR3 IGF-I/IGF-1 Protein, CF
Product Specific Notices for Recombinant Human LR3 IGF-I/IGF-1 Protein, CF
For research use only

