Recombinant Cynomolgus Monkey VSIG4 Fc Chimera Protein, CF
R&D Systems, part of Bio-Techne | Catalog # 10155-VS

Key Product Details
- R&D Systems HEK293-derived Recombinant Cynomolgus Monkey VSIG4 Fc Chimera Protein (10155-VS)
- Quality control testing to verify active proteins with lot specific assays by in-house scientists
- All R&D Systems proteins are covered with a 100% guarantee
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
Source
| Cynomolgus Monkey VSIG4 (Arg25-Pro288) Accession # XP_005593850.1 |
IEGRMD | Human IgG1 (Pro100-Lys330) |
| N-terminus | C-terminus |
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
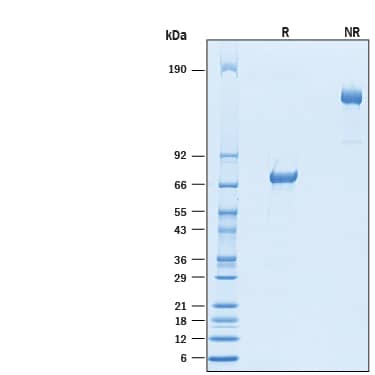
SDS-PAGE
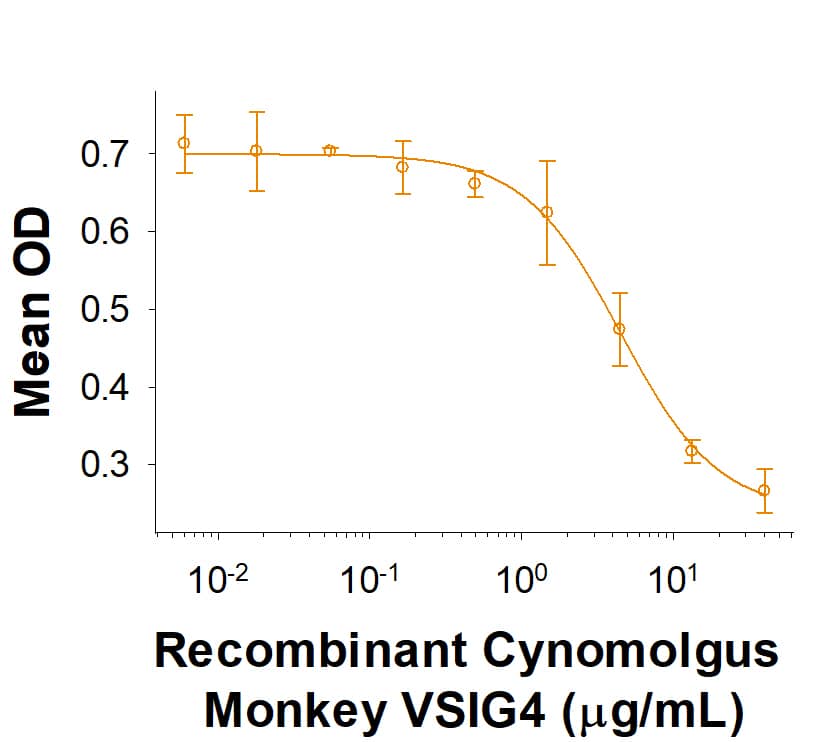
Activity
The ED50 for this effect is 0.6-6 μg/mL.
Scientific Data Images for Recombinant Cynomolgus Monkey VSIG4 Fc Chimera Protein, CF
Recombinant Cynomolgus Monkey VSIG4 Fc Chimera Protein Bioactivity
Recombinant Cynomolgus Monkey VSIG4 Fc Chimera (Catalog # 10155-VS) inhibits anti-CD3 antibody induced IFN-gamma secretion by human T cells. The ED50 for this effect is 0.6-6 μg/mL.Recombinant Cynomolgus Monkey VSIG4 Fc Chimera Protein SDS-PAGE
2 μg/lane of Recombinant Cynomolgus Monkey VSIG4 Fc Chimera (Catalog # 10155-VS) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie Blue staining, showing bands at 67-76 kDa and 130-150 kDa, respectively.Formulation, Preparation and Storage
10155-VS
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 200 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: VSIG4
VSIG4 (V-set and immunoglobulin domain containing 4), also known as CRIg and Z39IG, is a 45 kDa, type I transmembrane protein of the B7 family within the Ig superfamily that is expressed only in tissue-resident macrophages (1-4). The cynomolgus VSIG4 cDNA encodes 404 amino acids (aa) including a 24 aa signal sequence, a 264 aa extracellular domain (ECD) containing a V-type and a C2-type Ig domain, a 23 aa transmembrane domain and a 93 aa cytoplasmic domain (5). The cynomolgus VSIG4 ECD shares 94% aa identity with human VSIG4 ECD. VSIG4 is specifically expressed on macrophages in the thymic medulla, peritoneum, alveoli, synovia, adipose and heart, liver Kupffer cells, placental Hofbauer cells, and atherosclerotic foam cells (1-4, 6-9). It is absent on infiltrating macrophages (8). VSIG4 is a complement receptor that binds C3b and iC3b fragments, internalizes them to recycling endosomes, and is recycled to the cell surface (4, 6). It contributes significantly to innate immunity by binding and phagocytosis of complement-opsonized invading pathogens (4, 8, 10). Binding of either native or recombinant soluble VSIG4 to C3b inhibits complement amplification through the alternative, but not classical, pathway (10, 11). VSIG4 is also a negative regulator of mouse and human T cell activation (2). Although VSIG4 engagement may activate NF kappaB and thus be pro-inflammatory in some cases, many of its activities are important in resolving, rather than initiating, inflammation (1, 2, 7, 10, 11).
References
- He, J.Q. et al. (2008) Mol. Immunol. 4041.
- Vogt, L. et al. (2006) J. Clin. Invest. 116:2817.
- Langnaese, K. et al. (2000) Biochim. Biophys. Acta 1492:522.
- Helmy, K. et al. (2006) Cell 124:915.
- Entrez protein Accession # A0A2K5TW73, XP_005593850.
- Tanaka, M. et al. (2008) Clin. Exp. Immunol. 154:38.
- Lee, M-Y. et al. (2006) J. Leukoc. Biol. 80:922.
- Gorgani, N.N. et al. (2008) J. Immunol. 181:7902.
- Walker, M.G. (2002) Biochim. Biophys. Acta 1574:387.
- Wiesmann, C. et al. (2006) Nature 444:217.
- Katschke, K.J. et al. (2007) J. Exp. Med. 204:1319.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional VSIG4 Products
Product Documents for Recombinant Cynomolgus Monkey VSIG4 Fc Chimera Protein, CF
Product Specific Notices for Recombinant Cynomolgus Monkey VSIG4 Fc Chimera Protein, CF
For research use only