Human Erythropoietin Quantikine IVD ELISA Kit
R&D Systems, part of Bio-Techne | Catalog # DEP00B

Key Product Details
Assay Length
Sample Type & Volume Required Per Well
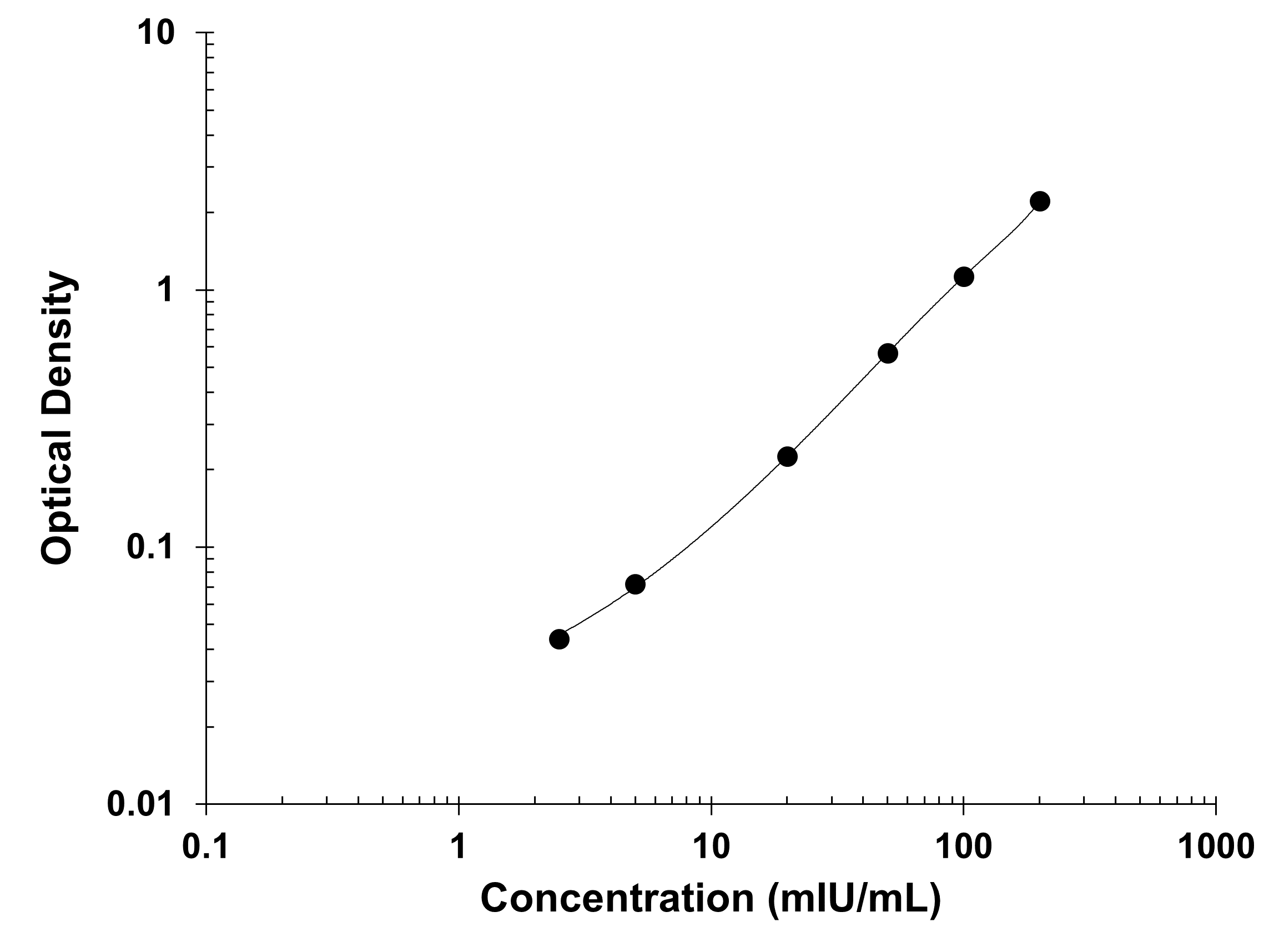
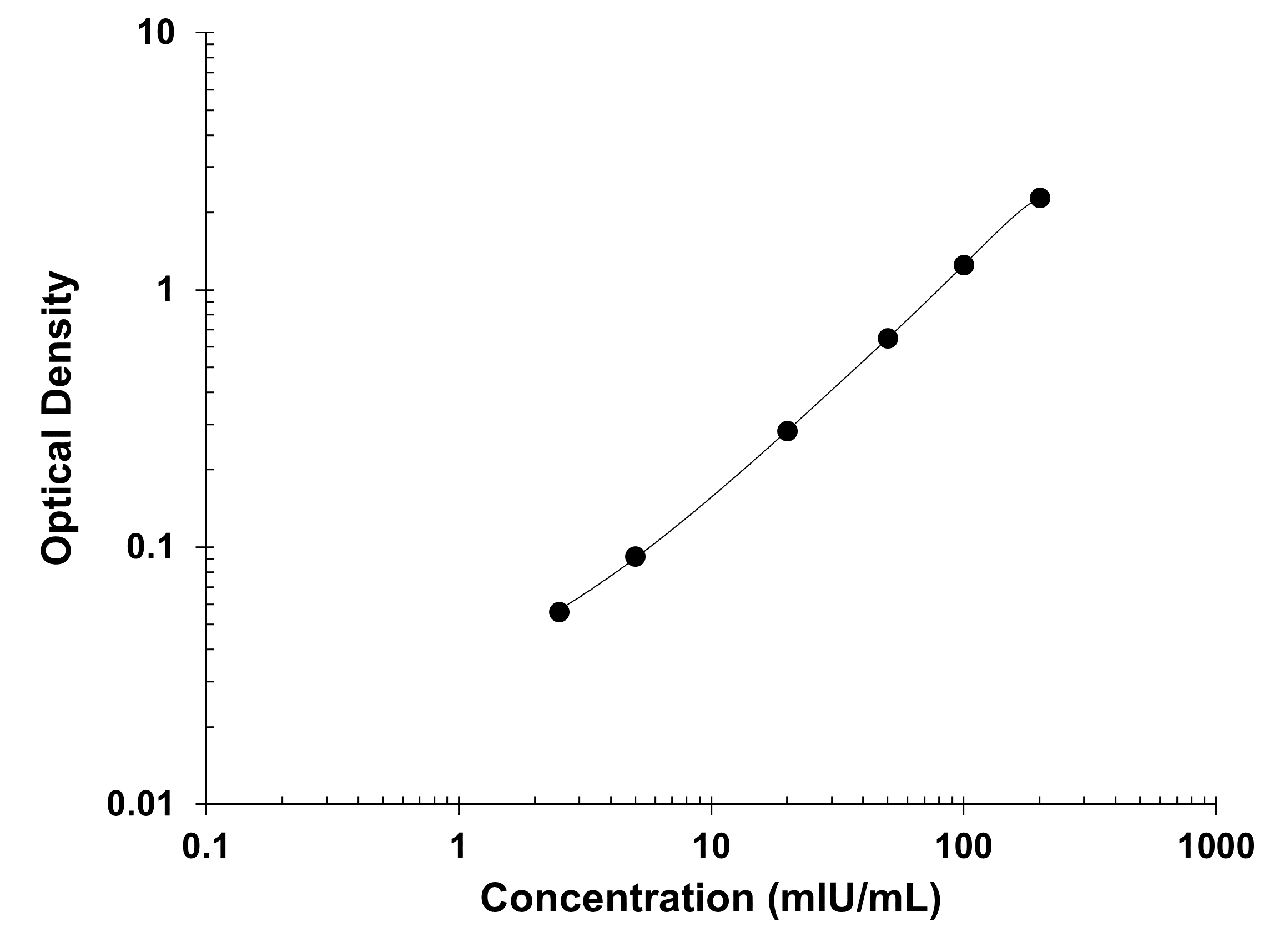
Sensitivity
Assay Range
Product Summary for Human Erythropoietin Quantikine IVD ELISA Kit
Product Specifications
Assay Type
Format
Measurement
Detection Method
Conjugate
Reactivity
Specificity
Cross-reactivity
Interference
Sample Values
| Serum | EDTA plasma |
| 3.3-16.6 mlU/mL | 3.1-14.9 mlU/mL |
Patients suffering from polycythemia rubra vera may have Epo concentrations within the normal range, whereas those suffering from secondary polycythemia may have elevated concentrations of serum Epo (1). Polycythemia rubra vera patients who undergo phlebotomy may have elevated serum Epo concentrations.
Precision
Intra-Assay Precision (Precision within an assay) Assay precision was assessed as outlined in the CLSI publication CLSI EP05-A3, Evaluation of Precision of Quantitative Measurement Procedures; Third Edition. The following modifications were made to the basic design described in the CLSI guideline. Three reagents lots were used instead of one. For within laboratory precision, 10 assays were run by 5 operators for each reagent lot over a period of at least 20 days with no more than 2 assays run per day. For within assay precision, 20 replicates of each control were run for each reagent lot. Three control pools were run in both the benchtop and shaker protocols of the Quantikine™ IVD Human Epo ELISA for both within laboratory and within assay precision. The results of each assay were tabulated and the within assay precision and within laboratory precision of each control pool was calculated. Benchtop ProtocolShaker Protocol Within Laboratory PrecisionWithin Assay PrecisionWithin Laboratory PrecisionWithin Assay PrecisionControl123123123123Mean (mIU/mL)9.4240.111610.641.31159.7940.91168.8139.4114n=303030202020303030202020%CV11.07.25.44.74.93.412.05.95.24.12.53.2
Inter-Assay Precision (Precision between assays)
Recovery for Human Erythropoietin Quantikine IVD ELISA Kit
Recovery was estimated by adding recombinant human Epo at three different levels across the range of the assay into six plasma and six serum specimens. Mean percent recoveries are shown in the table below:
| Benchtop Protocol | Shaker Protocol | |||
| Specimen Type | Average % Recovery | Range | Average % Recovery | Range |
| Serum (n=6) | 100 | 84-115 | 101 | 96-107 |
| EDTA Plasma (n=6) | 99 | 81-114 | 96 | 84-105 |
Linearity
To assess the linearity of the assay, samples spiked with high concentrations of Epo were serially diluted with Calibrator Diluent to produce samples with values within the dynamic range of the assay. Benchtop ProtocolShaker ProtocolSerumEDTA PlasmaSerumEDTA Plasma1:2Average % of Expected111110105109Range (%)106-118104-11899-109101-1171:4Average % of Expected109106102105Range (%)100-12394-12294-11395-1191:8Average % of Expected105999698Range (%)93-12392-11889-10689-114
Preparation and Storage
Shipping
Stability & Storage
Background: Erythropoietin/EPO
Erythropoietin/EPO is a renal glycoprotein hormone that promotes erythrocyte formation by preventing the apoptosis of early erythroid precursors which express the EPO receptor (EPO R). Rapid regulation of circulating EPO allows tight control of erythrocyte production and hemoglobin concentrations. Anemia or other causes of low tissue oxygen tension induce EPO production by stabilizing the hypoxia-induceable transcription factors HIF-1 alpha and HIF-2 alpha. Erythropoietin/EPO additionally plays a tissue-protective role in ischemia by blocking apoptosis and inducing angiogenesis.
R&D System products include Erythropoietin antibodies, proteins, and ELISAs. Our products cover multiple species and our EPO antibodies are validated for multiple applications including Neutralization, Western blot, ELISA, and Immunohistochemistry.