Human SIGIRR Antibody
R&D Systems, part of Bio-Techne | Catalog # AF990

Conjugate
Catalog #
Key Product Details
Species Reactivity
Validated:
Human
Cited:
Human, Mouse, Goat
Applications
Validated:
Western Blot
Cited:
Immunohistochemistry-Paraffin, Immunohistochemistry-Frozen, Western Blot, Flow Cytometry, Immunocytochemistry
Label
Unconjugated
Antibody Source
Polyclonal Goat IgG
Product Specifications
Immunogen
Mouse myeloma cell line NS0-derived recombinant human SIGIRR
Met1-His118
Accession # Q6IA17
Met1-His118
Accession # Q6IA17
Specificity
Detects human SIGIRR in direct ELISAs and Western blots. In direct ELISAs, less than 2% cross-reactivity with recombinant human (rh) IL‑1 R9, rhIL-1 R1, rhIL-1 R2, rhIL-1 R3, rhIL-1 R6, rhIL-1 R7, rhIL-1 R8, and rhIL-18 R is observed.
Clonality
Polyclonal
Host
Goat
Isotype
IgG
Scientific Data Images for Human SIGIRR Antibody
Detection of SIGIRR by Western Blot
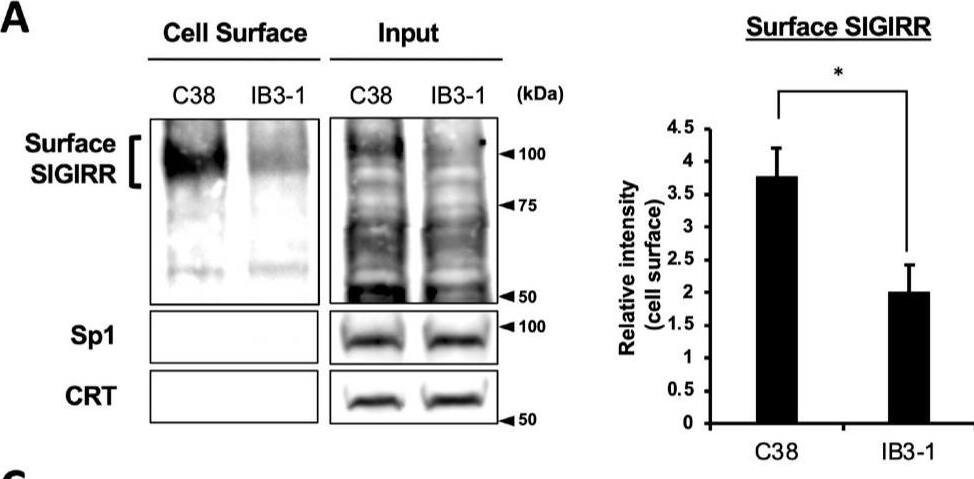
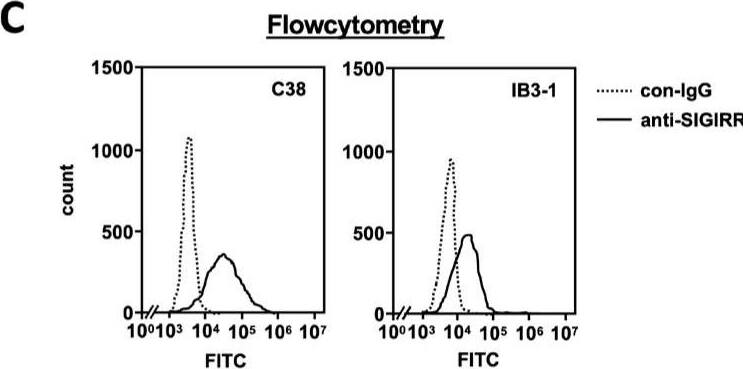
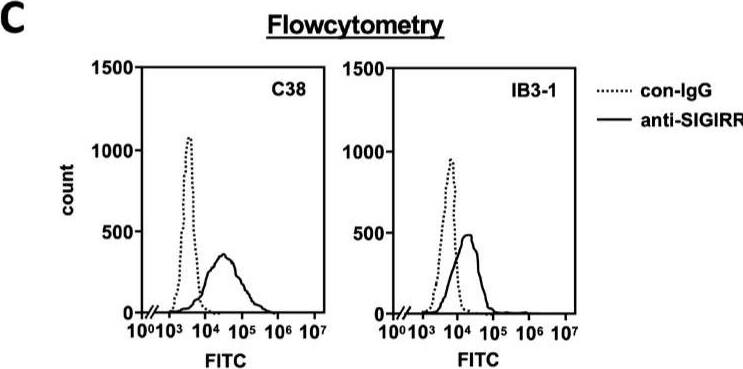
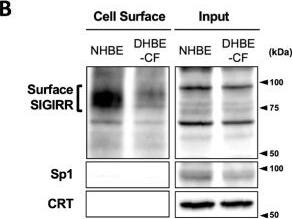
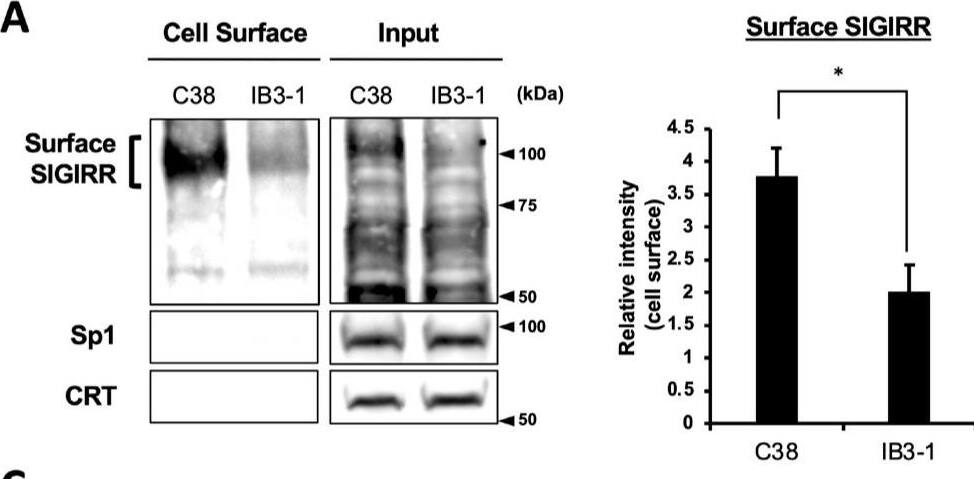
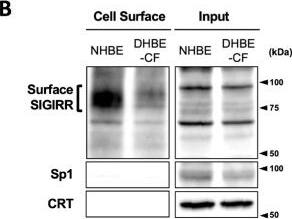
The expression of the cell surface of the SIGIRR is downregulated in CF cells. (A,B) Cell surface expression of the SIGIRR in (A) C38 and IB3-1, (B) NHBE and DHBE-CF (non-CF vs. CF) airway epithelial cells were assessed by biotinylation assay, followed by immunoblotting using anti-SIGIRR, calreticulin (CRT) and Sp1 antibodies. Protein bands in (A) were scanned, and relative band intensities were normalized to the beta-actin band. The graphs represent the average relative band intensity with S.D. from four independent experiments. * p < 0.05; Student’s t-test (n = 3). (C) Flow cytometry for the SIGIRR was performed using C38 and IB3-1 cells. Surface expressions of the SIGIRR were indicated by a fluorescence shift compared to the isotype control antibody (dotted line). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35887095), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of SIGIRR by Western Blot
The expression of the cell surface of the SIGIRR is downregulated in CF cells. (A,B) Cell surface expression of the SIGIRR in (A) C38 and IB3-1, (B) NHBE and DHBE-CF (non-CF vs. CF) airway epithelial cells were assessed by biotinylation assay, followed by immunoblotting using anti-SIGIRR, calreticulin (CRT) and Sp1 antibodies. Protein bands in (A) were scanned, and relative band intensities were normalized to the beta-actin band. The graphs represent the average relative band intensity with S.D. from four independent experiments. * p < 0.05; Student’s t-test (n = 3). (C) Flow cytometry for the SIGIRR was performed using C38 and IB3-1 cells. Surface expressions of the SIGIRR were indicated by a fluorescence shift compared to the isotype control antibody (dotted line). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35887095), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of SIGIRR by Western Blot
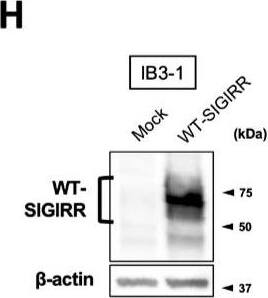
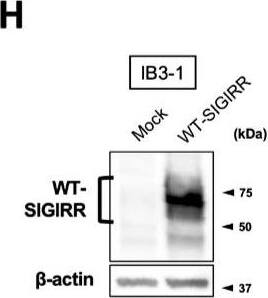
Suppression of IL-8 production by IL-37b is dependent on the cell surface-expressed WT-SIGIRR, leading to the attenuation of JNK phosphorylation. (A,B) C38 and IB3-1 cells were stimulated with 1 μg/mL flagellin or 20 μg/mL poly(I:C) 2 h after treatment with the indicated concentrations of a precursor or Val46 IL-37b. After 24 h, released IL-8 in the condition media was measured by ELISA. (C) NHBE and DHBE-CF cells were stimulated with 0.5 μg/mL poly(I:C) 2 h after treatment with 10 ng/mL of a precursor or Val46 IL-37b. After 24 h, released IL-8 in the condition media was measured by ELISA. (D) C38 and IB3-1 cells and (E) NHBE and DHBE-CF cells were stimulated with poly(I:C) (D, 20 μg/mL; E, 1 μg/mL) 2 h after treatment with a 10 ng/mL precursor of IL-37b. After the indicated period of the poly(I:C) treatment, the total cell lysates were subjected to immunoblotting using antibodies against the phospho-active form of MAPKs, IRF3, and I kappaB alpha. (F,G) pcDNA or delta8-SIGIRR-transfected C38 cells and (H and I) pcDNA or the WT-SIGIRR-transfected IB3-1 cells were stimulated with 20 μg/mL poly(I:C) 2 h after treatment with the indicated concentrations of the precursor of IL-37b. After 24 h, released IL-8 in the condition media was measured by ELISA. Exogenously expressed delta8 and the WT-SIGIRR were assessed by immunoblotting with the anti-SIGIRR. For the immunoblotting experiments (D–F,H), beta-actin was used as a loading control. (A–C,G,I) Results represent the mean ± S.D. * p < 0.05, ** p < 0.01, *** p < 0.001 versus poly(I:C)-treated cells; ANOVA with Dunnett’s test (n = 3). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35887095), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Human SIGIRR Antibody
Application
Recommended Usage
Western Blot
0.1 µg/mL
Sample: Recombinant Human SIGIRR Fc Chimera (Catalog # 990-SG)
Sample: Recombinant Human SIGIRR Fc Chimera (Catalog # 990-SG)
Formulation, Preparation, and Storage
Purification
Antigen Affinity-purified
Reconstitution
Reconstitute at 0.2 mg/mL in sterile PBS. For liquid material, refer to CoA for concentration.
Formulation
Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. *Small pack size (SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Shipping
Lyophilized product is shipped at ambient temperature. Liquid small pack size (-SP) is shipped with polar packs. Upon receipt, store immediately at the temperature recommended below.
Stability & Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: SIGIRR
References
- Thomassen, E. et al. (1999) Cytokine 11:389.
Long Name
Single lg IL-1 Related Protein
Alternate Names
IL-1R8, TIR8
Gene Symbol
SIGIRR
UniProt
Additional SIGIRR Products
Product Documents for Human SIGIRR Antibody
Product Specific Notices for Human SIGIRR Antibody
For research use only
Loading...
Loading...
Loading...
Loading...