Human Pappalysin-1/PAPP-A Antibody
R&D Systems, part of Bio-Techne | Catalog # AF2487

Key Product Details
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Glu82-Asp1214
Accession # Q13219
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human Pappalysin-1/PAPP-A Antibody
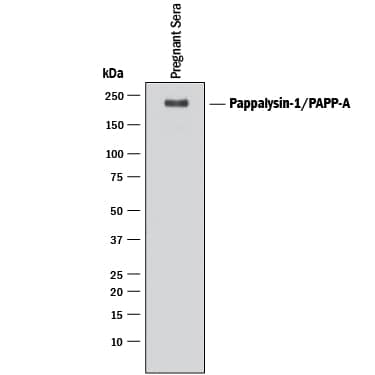
Detection of Human Pappalysin‑1/ PAPP‑A by Western Blot.
Western blot shows lysate of human pregnant sera. PVDF membrane was probed with 0.1 µg/mL of Goat Anti-Human Pappalysin-1/PAPP-A Antigen Affinity-purified Polyclonal Antibody (Catalog # AF2487) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for Pappalysin-1/PAPP-A at approximately 200 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.Detection of Human Pappalysin-1/PAPP-A by Western Blot
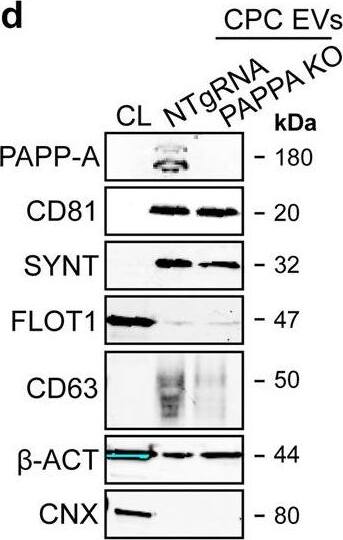
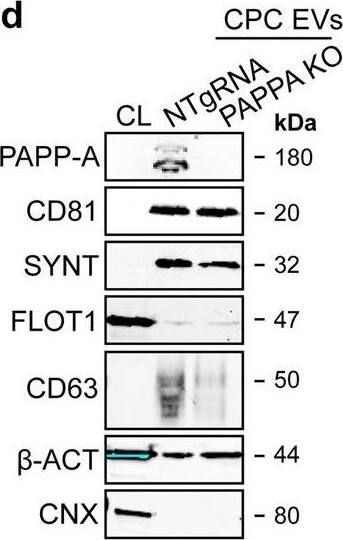
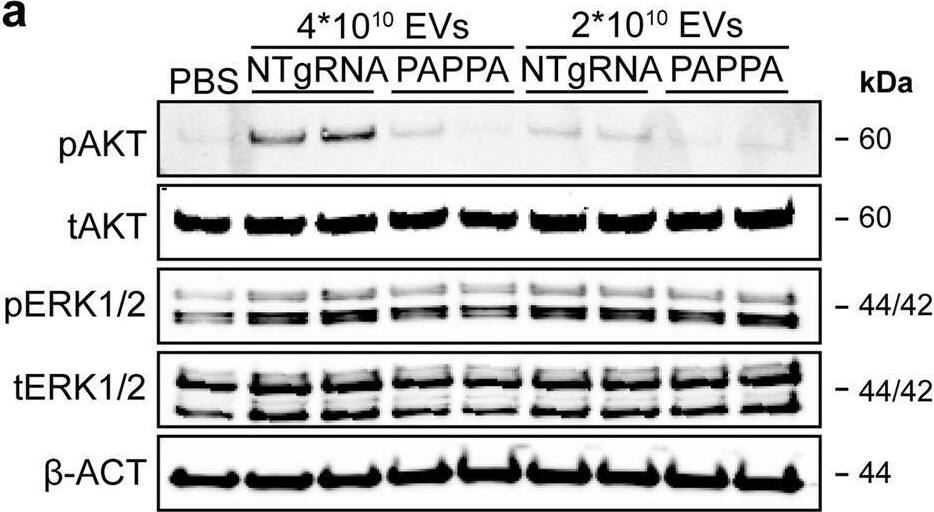
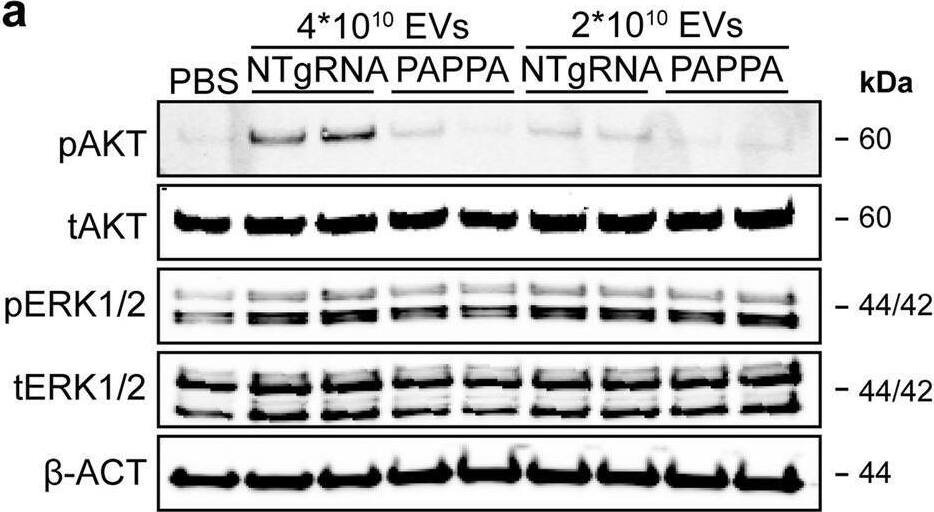
PAPPA KO-EVs were generated using CRISPR/Cas9.a Schematic depicting hypothesized mechanism of (intra)cellular signalling activated by EV-associated PAPP-A, based on identified proteins and significantly altered phosphosites measured in HMEC-1 upon veh-EV stimulation by (phopho)proteomic analysis. Detected proteins in HMEC-1 are displayed in grey, while significantly changing phosphosites present in cluster C1 (see Fig. 3d) are displayed in brown. b Representative western blot analysis of phosphorylated AKT (pAKT), total AKT (tAKT), phosphorylated ERK1/2 (pERK1/2) and total ERK1/2 (tERK1/2) in HMEC-1 treated with 6 × 1010 or 2 × 1010 CPC-EVs, or with 200 ng/mL free IGF-1 after pre-incubation with different doses of picropodophyllin (PPP). beta-actin ( beta-ACT) was included as housekeeping protein (I = phosphorylated protein blot, II = total protein blot). Biological replicates of (b) are displayed in Supplementary Fig. 9g, h. c Sanger sequencing results confirming 1 bp insertion in exon 3 of PAPPA at the CRISPR/Cas9 target site of the PAPPA KO-CPC clone, compared with the NTgRNA polyclonal CPC line. d Western blot analysis showing the absence of PAPP-A in PAPPA KO-EVs, compared with NTgRNA-EVs; the presence of CD81, CD63, Syntenin-1 (SYNT), Flotillin (FLOT1), beta-ACT, and absence of Calnexin (CNX) in both EV populations. FLOT1, beta-ACT and CNX were present in CPC lysate (CL). e Representative NTA plot showing the size distribution and particle concentration of PAPPA KO- and NTgRNA-CPC-EVs. f Protein content per 1 × 1010 PAPPA KO- and NTgRNA-EVs of two representative experiments. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37528162), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of Human Pappalysin-1/PAPP-A by Western Blot
PAPPA KO-EVs were generated using CRISPR/Cas9.a Schematic depicting hypothesized mechanism of (intra)cellular signalling activated by EV-associated PAPP-A, based on identified proteins and significantly altered phosphosites measured in HMEC-1 upon veh-EV stimulation by (phopho)proteomic analysis. Detected proteins in HMEC-1 are displayed in grey, while significantly changing phosphosites present in cluster C1 (see Fig. 3d) are displayed in brown. b Representative western blot analysis of phosphorylated AKT (pAKT), total AKT (tAKT), phosphorylated ERK1/2 (pERK1/2) and total ERK1/2 (tERK1/2) in HMEC-1 treated with 6 × 1010 or 2 × 1010 CPC-EVs, or with 200 ng/mL free IGF-1 after pre-incubation with different doses of picropodophyllin (PPP). beta-actin ( beta-ACT) was included as housekeeping protein (I = phosphorylated protein blot, II = total protein blot). Biological replicates of (b) are displayed in Supplementary Fig. 9g, h. c Sanger sequencing results confirming 1 bp insertion in exon 3 of PAPPA at the CRISPR/Cas9 target site of the PAPPA KO-CPC clone, compared with the NTgRNA polyclonal CPC line. d Western blot analysis showing the absence of PAPP-A in PAPPA KO-EVs, compared with NTgRNA-EVs; the presence of CD81, CD63, Syntenin-1 (SYNT), Flotillin (FLOT1), beta-ACT, and absence of Calnexin (CNX) in both EV populations. FLOT1, beta-ACT and CNX were present in CPC lysate (CL). e Representative NTA plot showing the size distribution and particle concentration of PAPPA KO- and NTgRNA-CPC-EVs. f Protein content per 1 × 1010 PAPPA KO- and NTgRNA-EVs of two representative experiments. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37528162), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Human Pappalysin-1/PAPP-A Antibody
Immunoprecipitation
Sample: Conditioned cell culture medium spiked with Recombinant Human Pappalysin-1/PAPP-A (Catalog # 2487-ZN), see our available Western blot detection antibodies
Western Blot
Sample: Human pregnant sera
Reviewed Applications
Read 1 review rated 5 using AF2487 in the following applications:
Formulation, Preparation, and Storage
Purification
Reconstitution
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Pappalysin-1/PAPP-A
Pappalysins belong to a fifth family of metzincins that consists of ADAMs/ADAMTSs, MMPs, astacins and serrylysins (1). PAPP-A is an important pregnancy protein and increases in plasma by a factor of about 150 during pregnancy as compared to the nonpregnant state. PAPP-A is also a major marker of Down syndrome in the first trimester of pregnancy because maternal serum levels of PAPP-A are significantly reduced when a fetus affected by Down syndrome is present (2). PAPP-A cleaves Insulin-like Growth Factor-Binding Protein-4 and -5 (IGFBP-4 and -5) at a single site, resulting in the release of bioactive IGF (3). Lack of IGFBP-4 cleavage in embryonic fibroblasts derived from PAPP-A knockout mice indicates that PAPP-A functions as a physiological IGFBP-4 protease (4). Three Lin12-Notch repeats (LNR) in the PAPP-A protein bind Ca2+ and are required for the cleavage of IGFBP-4, not IGFBP-5, by PAPP-A (5). The C-terminal LNR (residues 1476 to 1503) is not present in rhPAPP-A (residues 82 to 1214), which starts at the N-terminus of the mature chain and ends before the five Sushi (SCR) modules. As an active protease, rhPAPP-A cleaves IGFBP-5, which can be inhibited by 1,10-phenanthroline.
References
- Boldt, H.B. et al. (2001) Biochem. J. 358:359.
- Fialova L. and I.M. Malbohan (2002) Bratisl. Lek. Listy 103:194.
- Laursen, L.S. et al. (2001) FEBS Lett. 504:36.
- Conover, C.A. et al. (2004) Development 131:1187.
- Boldt, H.B. et al. (2004) J. Biol. Chem. 279:38525.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional Pappalysin-1/PAPP-A Products
Product Documents for Human Pappalysin-1/PAPP-A Antibody
Product Specific Notices for Human Pappalysin-1/PAPP-A Antibody
For research use only