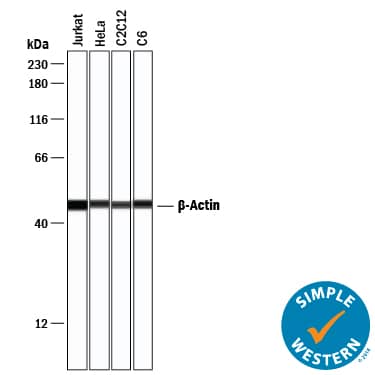
Detection of Human, Mouse, and Rat beta-Actin by Simple WesternTM.
Simple Western lane view shows lysates of Jurkat human acute T cell leukemia cell line, HeLa human cervical epithelial carcinoma cell line, C2C12 mouse myoblast cell line, and C6 rat glioma cell line, loaded at 0.2 mg/mL. A specific band was detected for beta-Actin at approximately 48 kDa (as indicated) using 1 µg/mL of Mouse Anti-Human/Mouse/Rat beta-Actin Monoclonal Antibody (Catalog # MAB8929) . This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
Detection of Human beta-Actin by Western Blot
The treatment of n-BP rescued the progression of QA-induced excitotoxicity in SCA3 PPs. The control and/or SCA3 PPs were treated with or without QA (1 μM) in the presence of n-BP (0, 10, 20, and 40 μg/mL) for 12 h. (A) Images of QA-treated SCA3 PPs in the presence of BP (40 μg/mL). (B) Confocal images of poly Q localization in BP (40 μg/mL)-treated SCA3 PPs in the presence of QA (1 um). When compared with Figure 2C, 40 μg/mL of n-BP prevented the amounts of colocalized polyQ in the cell nucleus of PPs. The distribution of polyQ in the nucleus is indicated by white arrowheads. Scale bar = 100 μm; (C) protein analysis of wild-type, mutant ATXN3 and its proteolytic fragment in cell lysates of control and SCA3 PPs, as assessed by immunoblots. Soluble, soluble protein; Insoluble, insoluble protein; (D,E) representative immunoblot of control and cleaved PARP1 in cell lysates of control and SCA3 PPs, as assessed by immunoblots. The HDAC2 were used as loading controls. The quantification from three independent images is presented as the means ± standard deviation. t(4) = 2.824, p < 0.05, for lane 1 vs. lane 2; t(4) = 5.613, p < 0.01, for lane 1 vs. lane 3; t(4) = 3.522, p < 0.05, for lane 1 vs. lane 4; (F) the quantification result of calcium concentration in SCA3 PPs, as assessed by Fura-2 indicator. Cells were treated with QA (1 μM) in the presence of n-BP (0, 10, 20, and 40 μg/mL). Data are presented as the means ± standard deviation. t(4) = 2.95, p < 0.05; (G) the calpain activity in control and SCA3 PPs cell lysates, as assessed by ELISA assay. The quantification results from three independent replicates are presented as the means ± standard deviation. t(4) = 3.27, p < 0.05, for lane 3 vs. lane 4; t(4) = 17.55, p < 0.01, for lane 3 vs. lane 5; t(4) = 9.019, p < 0.01, for lane 3 vs. lane 6. (H) Protein analysis of calpain 1, calpain 2 and calpastatin in cell lysates of SCA3 PPs, as assessed by immunoblots. Cells were treated with or without QA (1 μM) in the presence of n-BP (0, 10, 20, and 40 μg/mL). *, p < 0.05; **, p < 0.01. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35163312), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human beta-Actin by Simple Western
Identification of menin-associated proteins (MAPs) in breast cancer cells. (A) and (B) WES of BirA-Menin fusion proteins (A) and biotin-labeled proteins (B) in total lysates of BirA-MEN1 BioID engineered T47D or MCF-7 cells after incubating with or without doxycycline and biotin. (C) Schematic purification and proteomic identification of MAPs using LC–MS/MS. (D) Heatmap of the quantification of 35 MAPs commonly shared in T47D and MCF-7 cells. MAPs further verified by WES immunoassays were indicated by arrows. (E) Network analysis of 35 MAPs in MCF-7 cells. The distance between menin and MAPs represented the quantitative ratio of each MAP and menin. MAPs marked in blue were further assayed by WES. (F) Nuclear or cytoplasmic lysates of BirA-MEN1 BioID engineered T47D or MCF-7 cells after streptavidin beads pull-down were detected by WES with antibodies against menin, KMT2A, MED12, WAPL, GATA3, LaminA/C, or GAPDH. FL, full length; SP, spliced form. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32971831), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human beta-Actin by Simple Western
Identification of MEN1-modulated genes in breast cancer cells. (A) RT-qPCR of MEN1 in T47D or MCF-7 cells treated with vehicle or MEN1 shRNA lentivirus (n = 3). (B) Quantitative Western immunoassays (WES) of menin expression in T47D or MCF-7 cells treated with vehicle or MEN1 shRNA lentivirus (n = 3). (C) Venn diagrams of differentially expressed genes (fold change ≥1.5 or ≤0.66) in T47D or MCF-7 cells after shMEN1 knockdown compared with vehicle controls (n = 2). (D) Pathway annotation analysis of MEN1-upregulated and MEN1-downregulated genes in T47D or MCF-7 cells using DAVID including cancer hallmark pathways. (E) Schematic illustration of five major metabolic pathways. (F) Expression heat maps of oxidative phosphorylation (OXPHOS) and glycolytic genes in both MEN1 knockdown T47D and MCF-7 cells (fold changes relative to vehicle controls). (G) Bar charts of the expression levels of representative OXPHOS and glycolytic genes affected by MEN1 knockdown in T47D or MCF-7 cells using RT-qPCR. Data are presented as mean ± S.D. Unpaired two-tailed Student’s t-test was used for statistics. * p < 0.05, ** p < 0.01, and *** p < 0.001. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32971831), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Rat beta-Actin by Western Blot
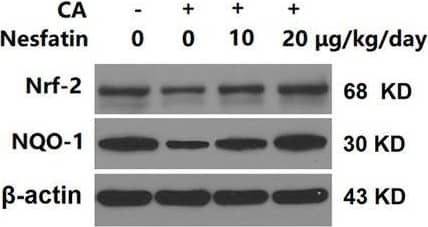
Nesfatin promoted nuclear translocation of Nrf‐2 with decreased cellular ROS levels. (A) The levels of Nrf‐2 and NQO‐1 in cytoplasmic fractions as measured by western blot analysis. (B) The levels of Nrf‐2 and NQO‐1 in nuclear fractions as measured by western blot analysis (**, p < 0.01 vs. vehicle group, #, ##, p < 0.05, 0.01 vs. CA group). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/39097921), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human beta-Actin by Western Blot
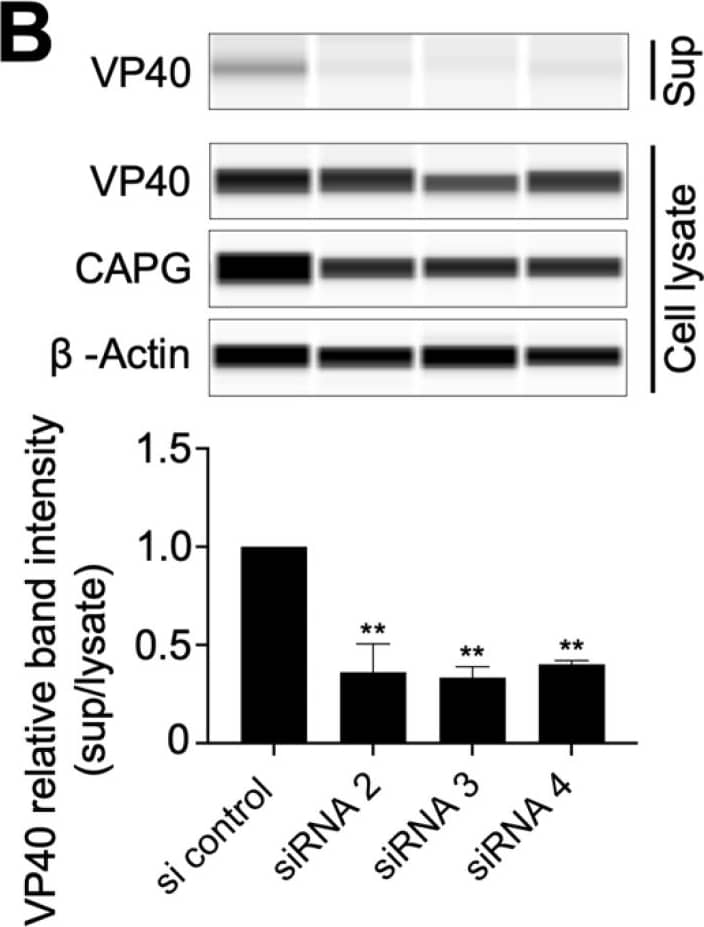
Effect of CAPG suppression on EBOV release from cells. (A) Measurement of the quantity of viral RNA released from siRNA treated cells. At 48 hpi, RNA was extracted from the supernatant (sup) and the remaining cells (cell lysate), then virus RNA levels measured by RT-qPCR using primers for NP. The graph indicates Cq supernatant-cell lysate signals in each sample relative to the siRNA control. (B) The efficiency of VLP formation from cells treated with each indicated siRNA. Hela cells seeded in a 6 well plate were transduced with siRNA (40 nM each) and pCAGGS-Ebola VP40 plasmid (0.5 µg). At 48 h post transfection, the supernatant was collected and centrifuged to remove cell debris. VLPs were collected by pelleting through a 20% sucrose cushion. VLP pellets and cell lysates were analyzed by immunoblot. Band intensity from each sample is shown relative to siRNA non-targeting control. All assays were repeated at least twice and the representative data sets are shown. One-way ANOVA with Dunnett’s multiple comparisons test was used for statistical analysis relative to control samples. One-way ANOVA with Dunnett’s multiple comparisons test was used for statistical analysis relative to control samples. The means at least two independent experiments ± SDs are shown. **, p < 0.01; ****, p < 0.0001. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36146710), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of beta-Actin by Western Blot
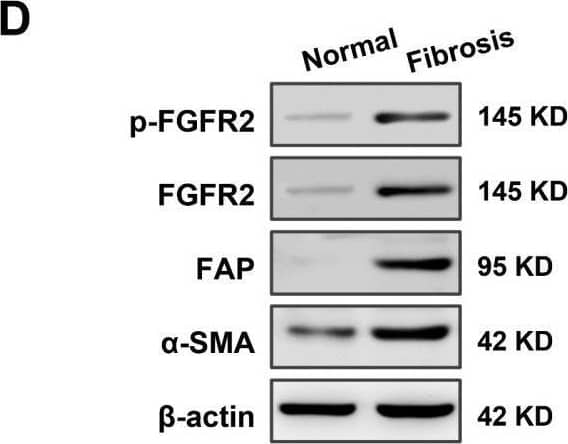
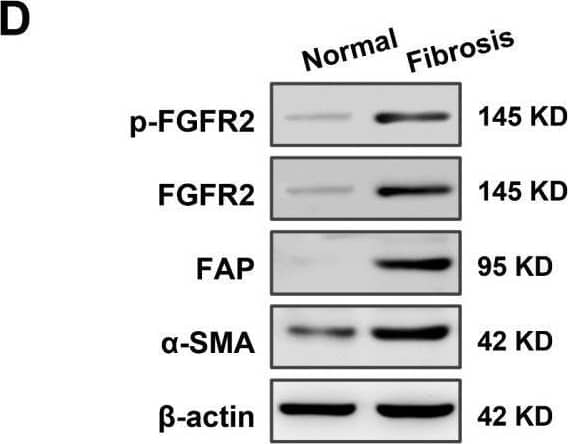
High expression of fibroblast growth factor receptor 2 (FGFR2) coincides with liver fibrosis. (A) The expression of FGFR2 in fibrotic liver and normal liver tissues was evaluated through data mining of human samples affected by Hepatitis B infection (GSE38941), alcohol abuse (GSE28619), and nonalcoholic steatohepatitis (GSE48452), as well as mouse samples induced with carbon tetrachloride (CCl4) (GSE152329, GSE98577). The differential fold expression was determined by comparing the expression of FGFR2 in the fibrotic liver and normal liver tissues. (B) A comparison of changes in FGFR2 expression in liver fibrosis patients in remission (improved) or not in remission (not improved) with mining data of GSE175448. (C) The correlation between FGFR2 expression and the liver fibrosis score (Ishak score) was analyzed by comparing FGFR2 expression in three pairs of liver fibrotic tissues and normal liver tissues through immunohistochemistry. (D) FGFR2 expression in liver fibrosis tissues and normal liver tissues was verified by Western blot and (E) q-PCR analyses. (E) Liver tissues from mice treated with CCl4 for different durations were used to determine the expression of the liver fibrosis markers Actin Alpha 2 (ACTA2) and FGFR2 by q-PCR and the trends of their expression with increasing days of induction. (F) The expression of ACTA2 and (G) FGFR2 was determined by q-PCR in liver tissues from mice treated with CCl4 for varying durations. The statistical significance was determined based on the p-value. The results are marked as significant “*” when p < 0.05, “**” when p < 0.01, “***” when p < 0.001, and not significant (ns) if p ≥ 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37111305), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human beta-Actin by Western Blot
Effect of CAPG suppression on EBOV release from cells. (A) Measurement of the quantity of viral RNA released from siRNA treated cells. At 48 hpi, RNA was extracted from the supernatant (sup) and the remaining cells (cell lysate), then virus RNA levels measured by RT-qPCR using primers for NP. The graph indicates Cq supernatant-cell lysate signals in each sample relative to the siRNA control. (B) The efficiency of VLP formation from cells treated with each indicated siRNA. Hela cells seeded in a 6 well plate were transduced with siRNA (40 nM each) and pCAGGS-Ebola VP40 plasmid (0.5 µg). At 48 h post transfection, the supernatant was collected and centrifuged to remove cell debris. VLPs were collected by pelleting through a 20% sucrose cushion. VLP pellets and cell lysates were analyzed by immunoblot. Band intensity from each sample is shown relative to siRNA non-targeting control. All assays were repeated at least twice and the representative data sets are shown. One-way ANOVA with Dunnett’s multiple comparisons test was used for statistical analysis relative to control samples. One-way ANOVA with Dunnett’s multiple comparisons test was used for statistical analysis relative to control samples. The means at least two independent experiments ± SDs are shown. **, p < 0.01; ****, p < 0.0001. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36146710), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of beta-Actin by Western Blot
High expression of fibroblast growth factor receptor 2 (FGFR2) coincides with liver fibrosis. (A) The expression of FGFR2 in fibrotic liver and normal liver tissues was evaluated through data mining of human samples affected by Hepatitis B infection (GSE38941), alcohol abuse (GSE28619), and nonalcoholic steatohepatitis (GSE48452), as well as mouse samples induced with carbon tetrachloride (CCl4) (GSE152329, GSE98577). The differential fold expression was determined by comparing the expression of FGFR2 in the fibrotic liver and normal liver tissues. (B) A comparison of changes in FGFR2 expression in liver fibrosis patients in remission (improved) or not in remission (not improved) with mining data of GSE175448. (C) The correlation between FGFR2 expression and the liver fibrosis score (Ishak score) was analyzed by comparing FGFR2 expression in three pairs of liver fibrotic tissues and normal liver tissues through immunohistochemistry. (D) FGFR2 expression in liver fibrosis tissues and normal liver tissues was verified by Western blot and (E) q-PCR analyses. (E) Liver tissues from mice treated with CCl4 for different durations were used to determine the expression of the liver fibrosis markers Actin Alpha 2 (ACTA2) and FGFR2 by q-PCR and the trends of their expression with increasing days of induction. (F) The expression of ACTA2 and (G) FGFR2 was determined by q-PCR in liver tissues from mice treated with CCl4 for varying durations. The statistical significance was determined based on the p-value. The results are marked as significant “*” when p < 0.05, “**” when p < 0.01, “***” when p < 0.001, and not significant (ns) if p ≥ 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37111305), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of beta-Actin by Western Blot
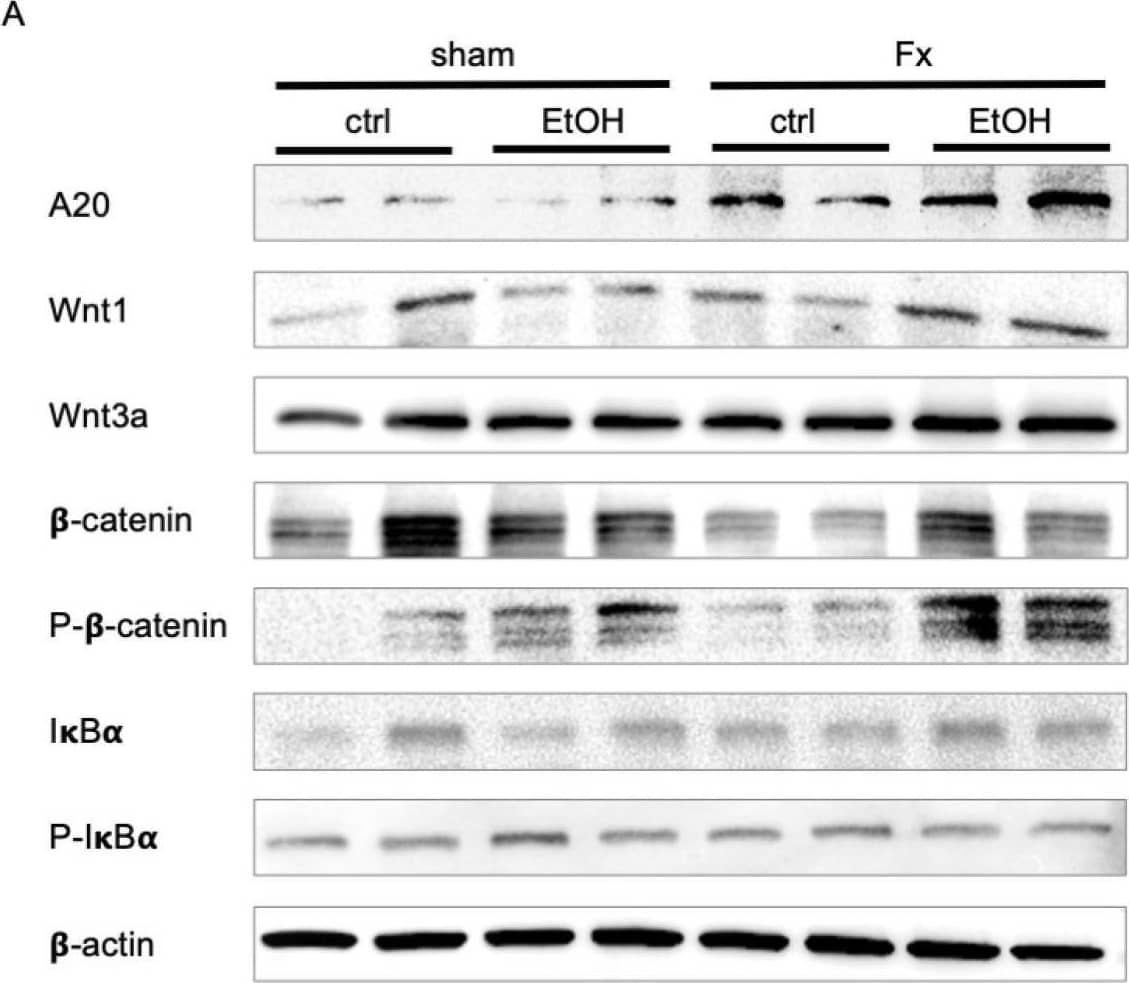
Regulation of Wnt and NF-kappa B signaling after acute alcohol intoxication (AAI) and fracture in the liver. Two hours before the initiation of experiments, the animals received an intragastric gavage of either sodium chloride (ctrl, n = 12) or ethanol (EtOH, n = 12) to simulate an AAI. Fx groups underwent osteotomy with the placement of an external fixator, and sham groups received only the external fixator. Twenty-four hours later, mice were euthanized, and liver sampling was performed. (A) Western blot analysis and quantification of protein expression levels of (B) A20, (C) Wnt1, (D) Wnt3a, (E) non-phosphorylated beta-catenin, and (F) phosphorylated beta-catenin, as well as (G) the ratio of phosphorylated and non-phosphorylated I kappaB alpha in liver tissue. The relative protein expression of normalized to beta-actin was calculated and is shown. * p < 0.05, n = 6 per group. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/40430063), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of beta-Actin by Western Blot
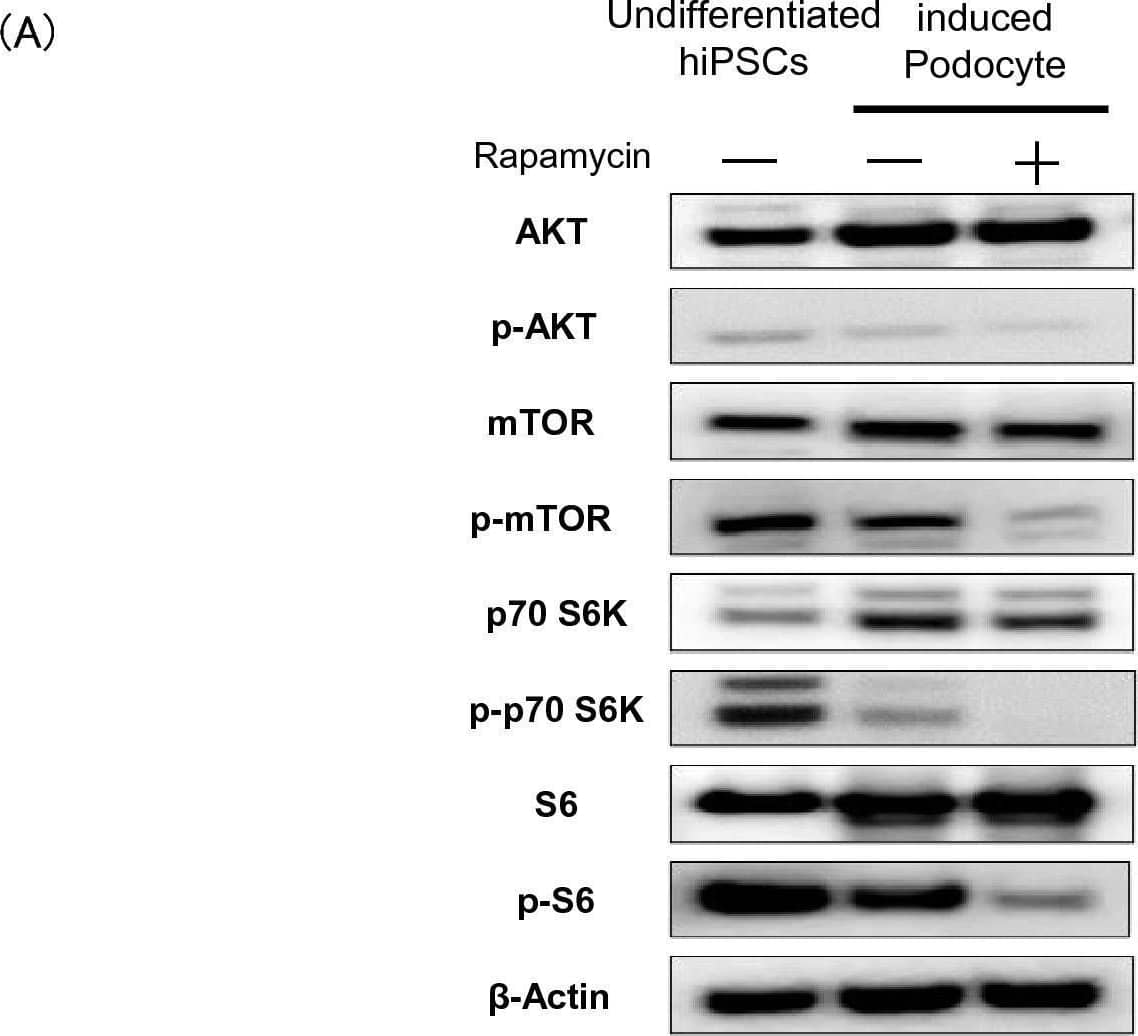
Importance of the mTOR pathway for podocyte differentiation. (A) Protein expression of mTOR, p-mTOR, p70 S6K, p-p70 S6K, S6, p-S6, AKT, and p-AKT, assessed using western blotting analysis. (B) mRNA expression of podocyte-associated genes (NEPHRIN, PODOCIN, SYNAPTOPODIN, WT1, and MAFB) following the addition of the S6 inhibitor LY2584702. Results are shown as the mean ± SD of 6 samples. Statistical analysis was performed using one-way ANOVA with Bonferroni’s test. ***p < 0.001. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37973990), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of beta-Actin by Western Blot
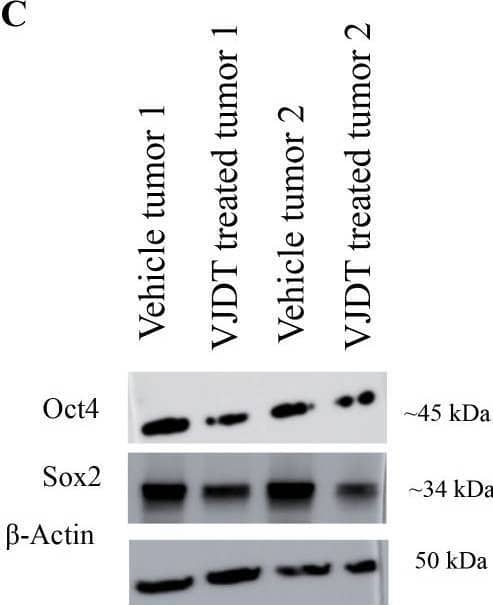
TREM1 inhibition via VJDT depletes LCSLCs, reduces tumor size, and decreases spheroid formation. (A) Tumor growth curves for Huh7 vehicle and VJDT treated mice (mean ± SEM, n=5 mice/group). Representative images of tumors from indicated groups on day 22. (B) Flow cytometry analysis shows a significant reduction in CD133+EpCAM+ LCSLCs in VJDT-treated tumors compared to the vehicle (n=4 per group). (C) Western blot analysis of two vehicle-treated and two VJDT-treated tumors shows reduced expression of stem cell-related proteins in VJDT-treated tumors. (D) Representative images from the spheroid formation assay demonstrate reduced spheroid formation following VJDT treatment. Scale bar = 50 µm. Spheroids were counted using ImageJ. **p<0.01, ***p<0.001, ns-not significant. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/40677705), licensed under a CC-BY license. Not internally tested by R&D Systems.