Detection of FGF basic/FGF2/bFGF by Western Blot
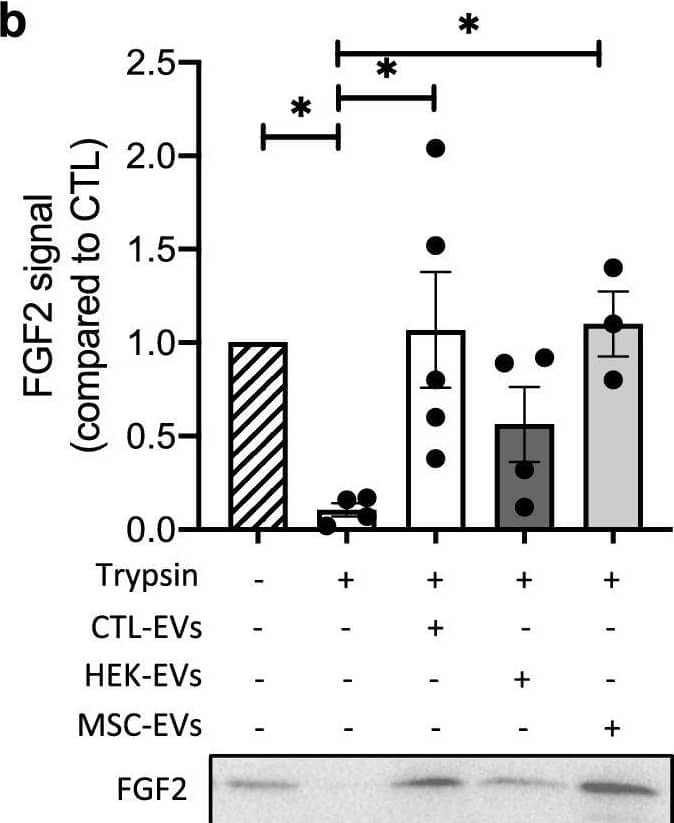
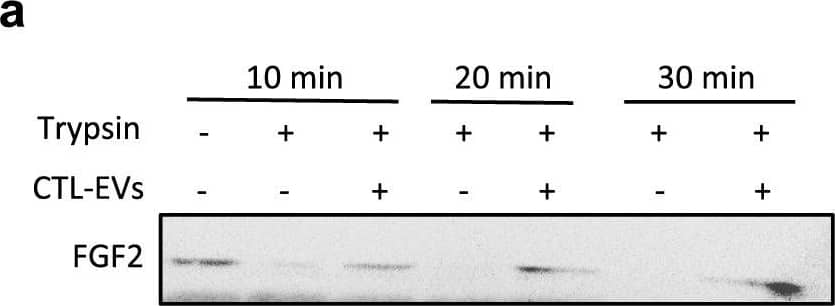
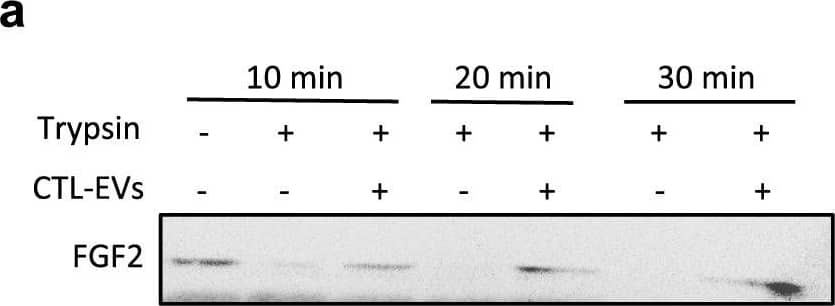
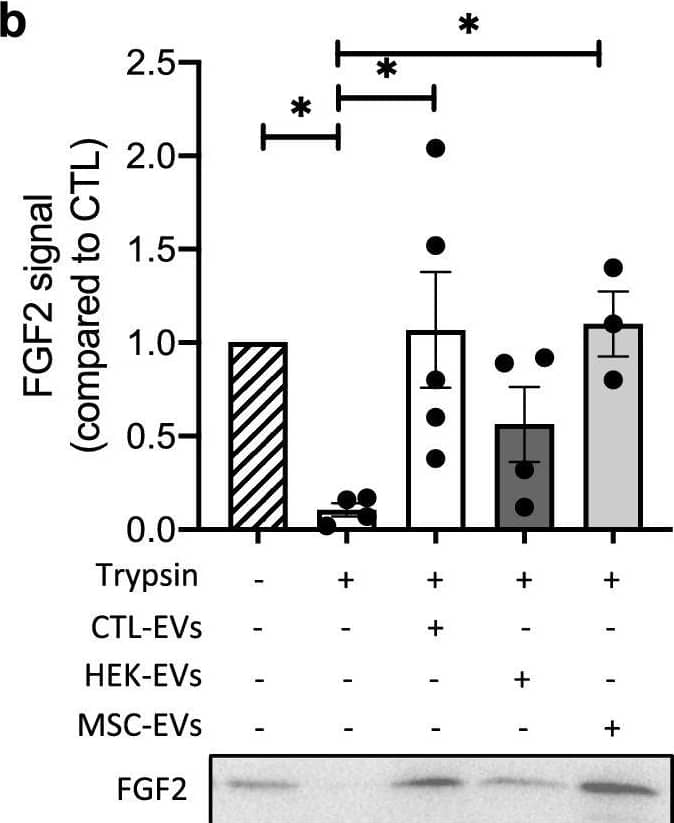
EV-bound FGF2 is protected from degradation. (A) Effect of EV binding on FGF2 thermal stability. FGF2 (10 ng/ml), alone or after incubation with EVs (8E + 8p) from different cell types for 1 h on ice, was placed at 37 °C for 24 h to challenge FGF2 thermal stability. Residual FGF2 activity was determined by (a) proliferation assay by BrDU incorporation and (b) ELISA assay to measure ESM-1 secretion. EVs from DF, MSC, HEK cells were tested. Results are mean ± SE of four independent experiments. Statistical significance was determined by one-way ANOVA (Dunnett’s multiple test), *< 0.05, **< 0.01, ***< 0.001, ****< 0.0001. (B) Effect of EV binding on FGF2 degradation by trypsin. FGF2 (10 ng), alone or after incubation with EVs from DF, MSC or HEK cells (8E + 8p) for 1 h on ice, was held at 37 °C with trypsin/EDTA (0.05%, diluted 1:2 v/v in 25 μl) for different time periods before loading on western blot to assess residual FGF2 protein. (a) Representative blot with CTL-EVs (b) Representative blot and quantification of FGF2 signal obtained after 10 min incubation with EVs from DF, MSC or HEK cells. Results are mean ± SE of 3–5 independent experiments. Statistical significance was determined by one-way ANOVA (Dunnett’s multiple test), *< 0.05. Original blots are shown in Suppl. Information. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36550142), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of FGF basic/FGF2/bFGF by Western Blot
EV-bound FGF2 is protected from degradation. (A) Effect of EV binding on FGF2 thermal stability. FGF2 (10 ng/ml), alone or after incubation with EVs (8E + 8p) from different cell types for 1 h on ice, was placed at 37 °C for 24 h to challenge FGF2 thermal stability. Residual FGF2 activity was determined by (a) proliferation assay by BrDU incorporation and (b) ELISA assay to measure ESM-1 secretion. EVs from DF, MSC, HEK cells were tested. Results are mean ± SE of four independent experiments. Statistical significance was determined by one-way ANOVA (Dunnett’s multiple test), *< 0.05, **< 0.01, ***< 0.001, ****< 0.0001. (B) Effect of EV binding on FGF2 degradation by trypsin. FGF2 (10 ng), alone or after incubation with EVs from DF, MSC or HEK cells (8E + 8p) for 1 h on ice, was held at 37 °C with trypsin/EDTA (0.05%, diluted 1:2 v/v in 25 μl) for different time periods before loading on western blot to assess residual FGF2 protein. (a) Representative blot with CTL-EVs (b) Representative blot and quantification of FGF2 signal obtained after 10 min incubation with EVs from DF, MSC or HEK cells. Results are mean ± SE of 3–5 independent experiments. Statistical significance was determined by one-way ANOVA (Dunnett’s multiple test), *< 0.05. Original blots are shown in Suppl. Information. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36550142), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of FGF basic/FGF2/bFGF by Western Blot
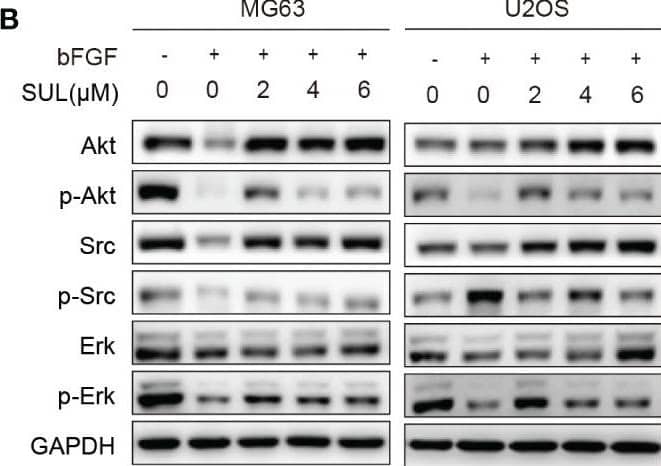
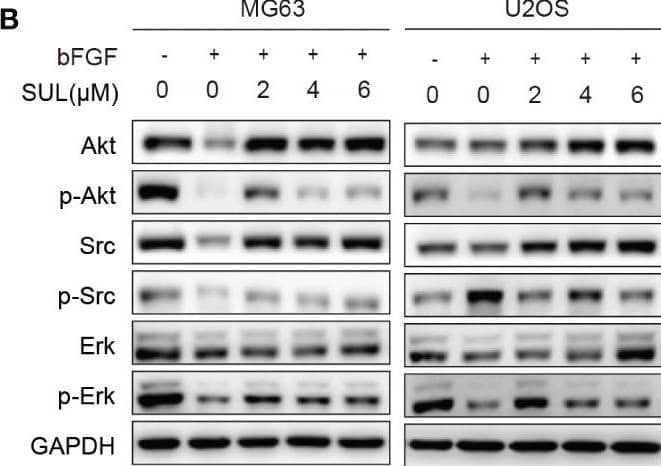
Sulfatinib inhibits OS migration through suppression of epithelial–mesenchymal transition (EMT). (A) The protein levels of EMT and metastatic markers were detected by Western blotting after 24 h sulfatinib treatment. (B) Sulfatinib inhibits phosphorylation of migration-related signal pathway induced by bFGF. (C) Immunohistochemistry for EMT- related markers (FGFR1, p-FGFR1, N-cadherin, and E-cadherin) in tumor sections after treatment (scale bar, 100 μm). OS, osteosarcoma; bFGF, basic fibroblast growth factor. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37361567), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of FGF basic/FGF2/bFGF by Western Blot
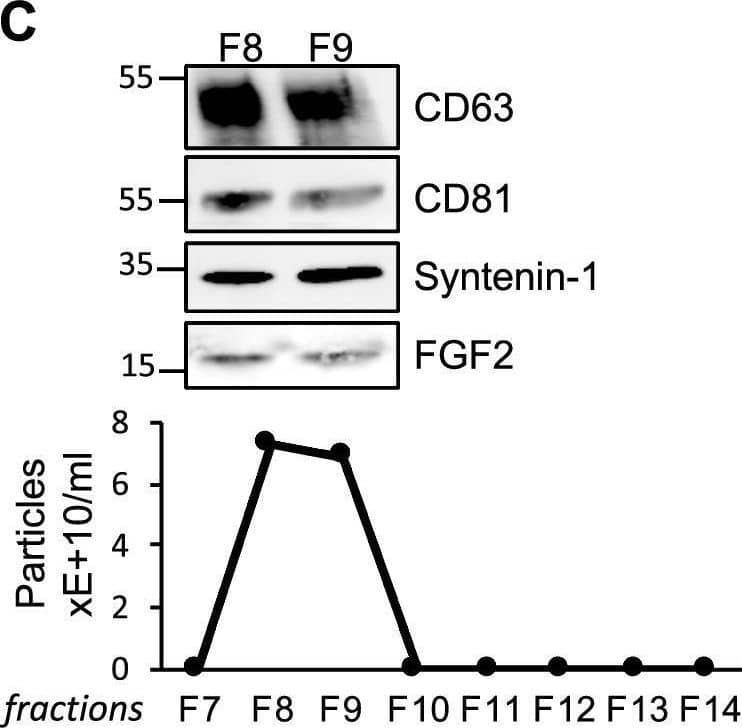
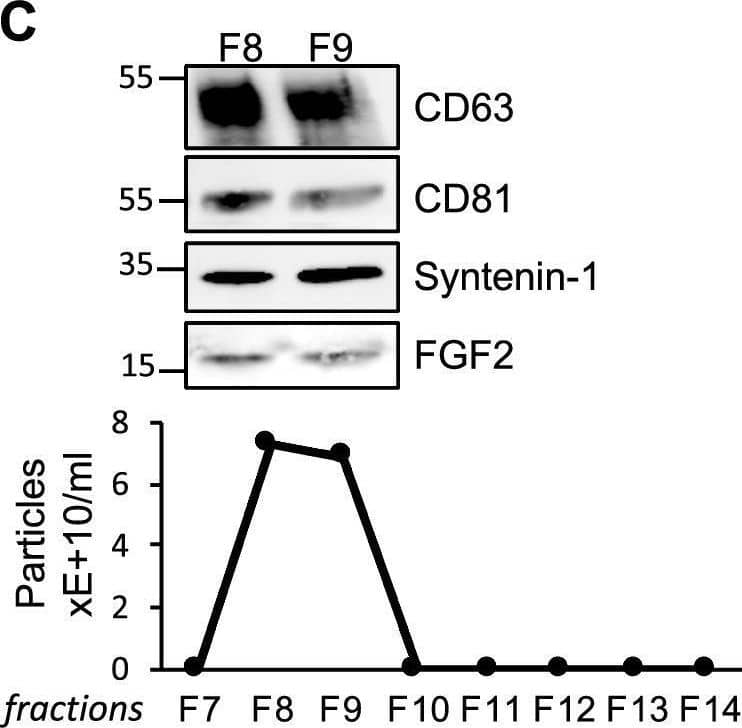
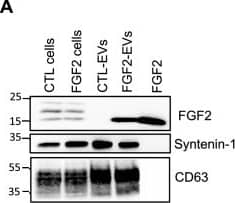
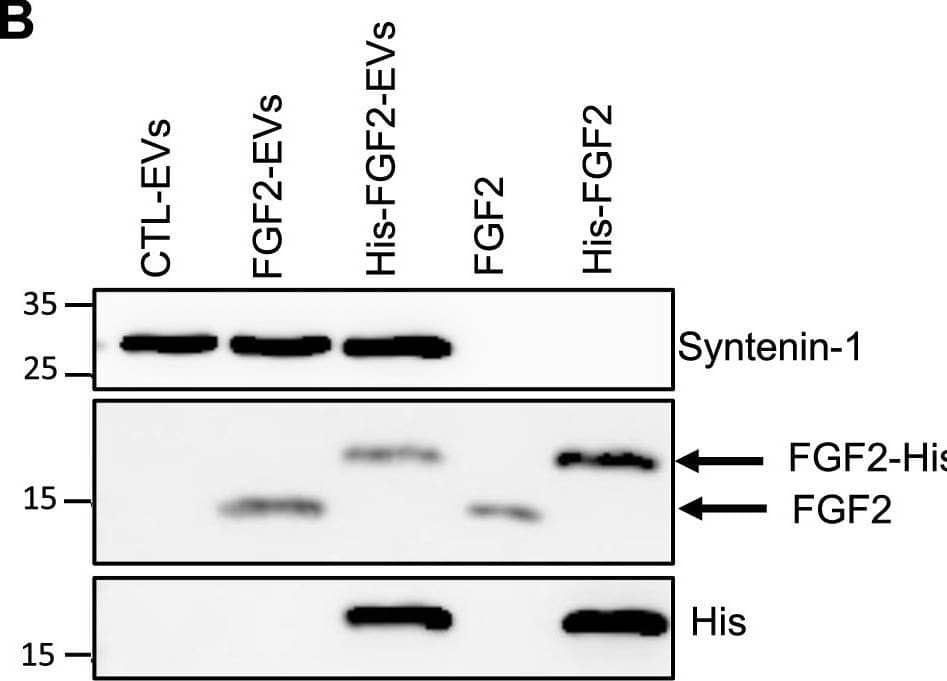
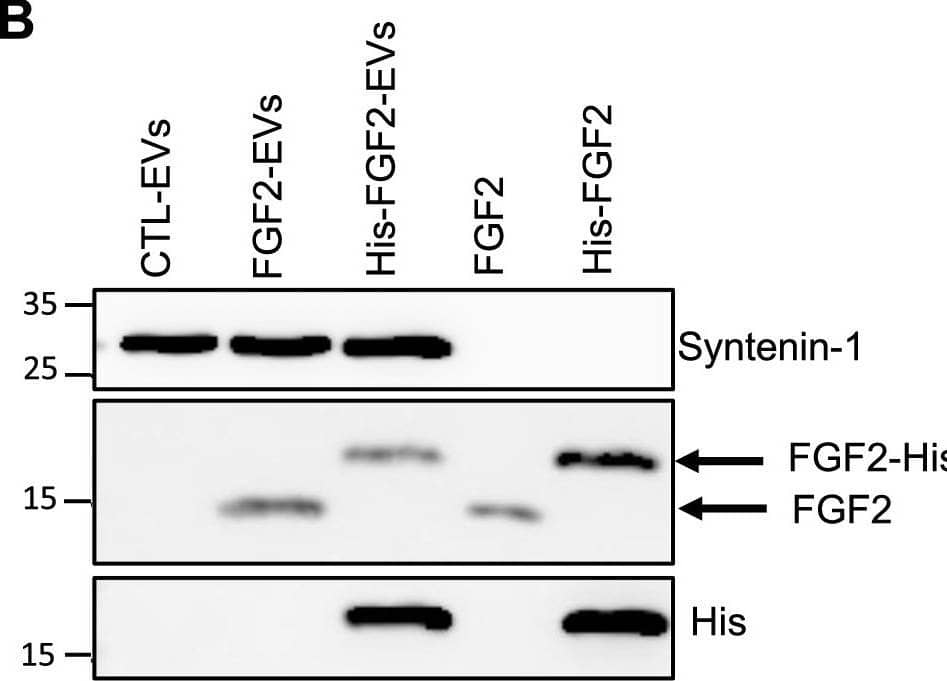
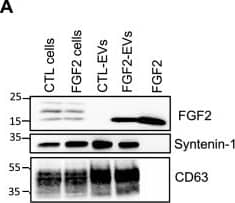
FGF2 is found at the outer surface of EVs secreted by dermal fibroblasts cultured in FGF2-containing medium. (A) FGF2, syntenin-1 and CD63 expression in DF cells and EV lysate (20 μg) was examined by western blot. Three isoforms of endogenous FGF2 were present in DF cells while FGF2-EVs contained the low molecular weight FGF2 isoform corresponding to recombinant FGF2. Original blots are shown in Suppl. Information. (B) Cells were cultured in the presence of His-tagged FGF2; western blot with 15 μg lysate and using anti-His antibody revealed the presence of His-FGF2 in secreted EVs. A shift in FGF2 size was also observed. Syntenin-1 was used as EV marker. Original blots are shown in Suppl. Information. (C) FGF2-EVs were loaded on a SEC column and collected fractions 8 and 9 (3E + 9p) were analyzed by western blot using antibodies for FGF2 and the EV markers CD63, CD81 and syntenin-1. Original blots are shown in Suppl. Information. For quantification, fractions were also analyzed by NTA. (D) FGF2 detection in FGF2-EVs by ELISA assay. Intact CTL- and FGF2-EVs (8E + 8p) were directly placed on FGF2 ELISA wells for external surface detection. FGF2-EVs were also treated with 0.1% triton and analyzed to detect internal FGF2. No FGF2 signal was detected in CTL-EVs. (E) FGF2 expression on FGF2-EVs was detected by flow cytometry. EVs were coupled to latex beads and labeled with FGF2 antibody before analysis by cytometry. Beads without EVs (beads) and beads labelled with goat IgG antibody (IgG) serve as negative controls. A representative plot is shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36550142), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of FGF basic/FGF2/bFGF by Western Blot
FGF2 is found at the outer surface of EVs secreted by dermal fibroblasts cultured in FGF2-containing medium. (A) FGF2, syntenin-1 and CD63 expression in DF cells and EV lysate (20 μg) was examined by western blot. Three isoforms of endogenous FGF2 were present in DF cells while FGF2-EVs contained the low molecular weight FGF2 isoform corresponding to recombinant FGF2. Original blots are shown in Suppl. Information. (B) Cells were cultured in the presence of His-tagged FGF2; western blot with 15 μg lysate and using anti-His antibody revealed the presence of His-FGF2 in secreted EVs. A shift in FGF2 size was also observed. Syntenin-1 was used as EV marker. Original blots are shown in Suppl. Information. (C) FGF2-EVs were loaded on a SEC column and collected fractions 8 and 9 (3E + 9p) were analyzed by western blot using antibodies for FGF2 and the EV markers CD63, CD81 and syntenin-1. Original blots are shown in Suppl. Information. For quantification, fractions were also analyzed by NTA. (D) FGF2 detection in FGF2-EVs by ELISA assay. Intact CTL- and FGF2-EVs (8E + 8p) were directly placed on FGF2 ELISA wells for external surface detection. FGF2-EVs were also treated with 0.1% triton and analyzed to detect internal FGF2. No FGF2 signal was detected in CTL-EVs. (E) FGF2 expression on FGF2-EVs was detected by flow cytometry. EVs were coupled to latex beads and labeled with FGF2 antibody before analysis by cytometry. Beads without EVs (beads) and beads labelled with goat IgG antibody (IgG) serve as negative controls. A representative plot is shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36550142), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of FGF basic/FGF2/bFGF by Western Blot
EV-bound FGF2 is protected from degradation. (A) Effect of EV binding on FGF2 thermal stability. FGF2 (10 ng/ml), alone or after incubation with EVs (8E + 8p) from different cell types for 1 h on ice, was placed at 37 °C for 24 h to challenge FGF2 thermal stability. Residual FGF2 activity was determined by (a) proliferation assay by BrDU incorporation and (b) ELISA assay to measure ESM-1 secretion. EVs from DF, MSC, HEK cells were tested. Results are mean ± SE of four independent experiments. Statistical significance was determined by one-way ANOVA (Dunnett’s multiple test), *< 0.05, **< 0.01, ***< 0.001, ****< 0.0001. (B) Effect of EV binding on FGF2 degradation by trypsin. FGF2 (10 ng), alone or after incubation with EVs from DF, MSC or HEK cells (8E + 8p) for 1 h on ice, was held at 37 °C with trypsin/EDTA (0.05%, diluted 1:2 v/v in 25 μl) for different time periods before loading on western blot to assess residual FGF2 protein. (a) Representative blot with CTL-EVs (b) Representative blot and quantification of FGF2 signal obtained after 10 min incubation with EVs from DF, MSC or HEK cells. Results are mean ± SE of 3–5 independent experiments. Statistical significance was determined by one-way ANOVA (Dunnett’s multiple test), *< 0.05. Original blots are shown in Suppl. Information. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36550142), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of FGF basic/FGF2/bFGF by Western Blot
Sulfatinib inhibits OS migration through suppression of epithelial–mesenchymal transition (EMT). (A) The protein levels of EMT and metastatic markers were detected by Western blotting after 24 h sulfatinib treatment. (B) Sulfatinib inhibits phosphorylation of migration-related signal pathway induced by bFGF. (C) Immunohistochemistry for EMT- related markers (FGFR1, p-FGFR1, N-cadherin, and E-cadherin) in tumor sections after treatment (scale bar, 100 μm). OS, osteosarcoma; bFGF, basic fibroblast growth factor. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37361567), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of FGF basic/FGF2/bFGF by Western Blot
FGF2 is found at the outer surface of EVs secreted by dermal fibroblasts cultured in FGF2-containing medium. (A) FGF2, syntenin-1 and CD63 expression in DF cells and EV lysate (20 μg) was examined by western blot. Three isoforms of endogenous FGF2 were present in DF cells while FGF2-EVs contained the low molecular weight FGF2 isoform corresponding to recombinant FGF2. Original blots are shown in Suppl. Information. (B) Cells were cultured in the presence of His-tagged FGF2; western blot with 15 μg lysate and using anti-His antibody revealed the presence of His-FGF2 in secreted EVs. A shift in FGF2 size was also observed. Syntenin-1 was used as EV marker. Original blots are shown in Suppl. Information. (C) FGF2-EVs were loaded on a SEC column and collected fractions 8 and 9 (3E + 9p) were analyzed by western blot using antibodies for FGF2 and the EV markers CD63, CD81 and syntenin-1. Original blots are shown in Suppl. Information. For quantification, fractions were also analyzed by NTA. (D) FGF2 detection in FGF2-EVs by ELISA assay. Intact CTL- and FGF2-EVs (8E + 8p) were directly placed on FGF2 ELISA wells for external surface detection. FGF2-EVs were also treated with 0.1% triton and analyzed to detect internal FGF2. No FGF2 signal was detected in CTL-EVs. (E) FGF2 expression on FGF2-EVs was detected by flow cytometry. EVs were coupled to latex beads and labeled with FGF2 antibody before analysis by cytometry. Beads without EVs (beads) and beads labelled with goat IgG antibody (IgG) serve as negative controls. A representative plot is shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36550142), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of FGF basic/FGF2/bFGF by Western Blot
FGF2 is found at the outer surface of EVs secreted by dermal fibroblasts cultured in FGF2-containing medium. (A) FGF2, syntenin-1 and CD63 expression in DF cells and EV lysate (20 μg) was examined by western blot. Three isoforms of endogenous FGF2 were present in DF cells while FGF2-EVs contained the low molecular weight FGF2 isoform corresponding to recombinant FGF2. Original blots are shown in Suppl. Information. (B) Cells were cultured in the presence of His-tagged FGF2; western blot with 15 μg lysate and using anti-His antibody revealed the presence of His-FGF2 in secreted EVs. A shift in FGF2 size was also observed. Syntenin-1 was used as EV marker. Original blots are shown in Suppl. Information. (C) FGF2-EVs were loaded on a SEC column and collected fractions 8 and 9 (3E + 9p) were analyzed by western blot using antibodies for FGF2 and the EV markers CD63, CD81 and syntenin-1. Original blots are shown in Suppl. Information. For quantification, fractions were also analyzed by NTA. (D) FGF2 detection in FGF2-EVs by ELISA assay. Intact CTL- and FGF2-EVs (8E + 8p) were directly placed on FGF2 ELISA wells for external surface detection. FGF2-EVs were also treated with 0.1% triton and analyzed to detect internal FGF2. No FGF2 signal was detected in CTL-EVs. (E) FGF2 expression on FGF2-EVs was detected by flow cytometry. EVs were coupled to latex beads and labeled with FGF2 antibody before analysis by cytometry. Beads without EVs (beads) and beads labelled with goat IgG antibody (IgG) serve as negative controls. A representative plot is shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36550142), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of FGF basic/FGF2/bFGF by Western Blot
EV-bound FGF2 is protected from degradation. (A) Effect of EV binding on FGF2 thermal stability. FGF2 (10 ng/ml), alone or after incubation with EVs (8E + 8p) from different cell types for 1 h on ice, was placed at 37 °C for 24 h to challenge FGF2 thermal stability. Residual FGF2 activity was determined by (a) proliferation assay by BrDU incorporation and (b) ELISA assay to measure ESM-1 secretion. EVs from DF, MSC, HEK cells were tested. Results are mean ± SE of four independent experiments. Statistical significance was determined by one-way ANOVA (Dunnett’s multiple test), *< 0.05, **< 0.01, ***< 0.001, ****< 0.0001. (B) Effect of EV binding on FGF2 degradation by trypsin. FGF2 (10 ng), alone or after incubation with EVs from DF, MSC or HEK cells (8E + 8p) for 1 h on ice, was held at 37 °C with trypsin/EDTA (0.05%, diluted 1:2 v/v in 25 μl) for different time periods before loading on western blot to assess residual FGF2 protein. (a) Representative blot with CTL-EVs (b) Representative blot and quantification of FGF2 signal obtained after 10 min incubation with EVs from DF, MSC or HEK cells. Results are mean ± SE of 3–5 independent experiments. Statistical significance was determined by one-way ANOVA (Dunnett’s multiple test), *< 0.05. Original blots are shown in Suppl. Information. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36550142), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of FGF basic/FGF2/bFGF by Western Blot
FGF2 is found at the outer surface of EVs secreted by dermal fibroblasts cultured in FGF2-containing medium. (A) FGF2, syntenin-1 and CD63 expression in DF cells and EV lysate (20 μg) was examined by western blot. Three isoforms of endogenous FGF2 were present in DF cells while FGF2-EVs contained the low molecular weight FGF2 isoform corresponding to recombinant FGF2. Original blots are shown in Suppl. Information. (B) Cells were cultured in the presence of His-tagged FGF2; western blot with 15 μg lysate and using anti-His antibody revealed the presence of His-FGF2 in secreted EVs. A shift in FGF2 size was also observed. Syntenin-1 was used as EV marker. Original blots are shown in Suppl. Information. (C) FGF2-EVs were loaded on a SEC column and collected fractions 8 and 9 (3E + 9p) were analyzed by western blot using antibodies for FGF2 and the EV markers CD63, CD81 and syntenin-1. Original blots are shown in Suppl. Information. For quantification, fractions were also analyzed by NTA. (D) FGF2 detection in FGF2-EVs by ELISA assay. Intact CTL- and FGF2-EVs (8E + 8p) were directly placed on FGF2 ELISA wells for external surface detection. FGF2-EVs were also treated with 0.1% triton and analyzed to detect internal FGF2. No FGF2 signal was detected in CTL-EVs. (E) FGF2 expression on FGF2-EVs was detected by flow cytometry. EVs were coupled to latex beads and labeled with FGF2 antibody before analysis by cytometry. Beads without EVs (beads) and beads labelled with goat IgG antibody (IgG) serve as negative controls. A representative plot is shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36550142), licensed under a CC-BY license. Not internally tested by R&D Systems.