Human CD3 GMP Antibody GMP
R&D Systems, part of Bio-Techne | Catalog # MAB11411-GMP
Recombinant Monoclonal Antibody. See GMP Antibody Protocols!

Key Product Details
Validated by
Biological Validation
Species Reactivity
Human
Applications
T Cell Stimulation
Label
Unconjugated
Antibody Source
Monoclonal Human IgG1 Clone # 28497-1
Product Specifications
Immunogen
Human T lymphocytes
Specificity
Detects human CD3 in a flow cytometry-based assay using Jurkat cells
Clonality
Monoclonal
Host
Human
Isotype
IgG1
Purity
≥95%, by SDS-PAGE with quantitative densitometry by Coomassie® Blue Staining.
Endotoxin Level
<0.10 EU per 1 μg of the antibody by the LAL method.
Host Cell Protein
≤0.500 ng per μg of antibody when tested by ELISA
Host Cell DNA
≤0.0100 ng per μg of antibody when tested by PCR
Mycoplasma
Negative when tested in a ribosomal RNA hybridization assay
Activity
Measured by its ability to induce IL-2 secretion by Jurkat human acute T cell leukemia cells. Freeman, G.J. et al. (1993) Science 262:909.
The ED50 for this effect is 8.00‑80.0 ng/mL
The ED50 for this effect is 8.00‑80.0 ng/mL
Aggregation
≤10% aggregation when tested by SEC
Scientific Data Images for Human CD3 GMP Antibody
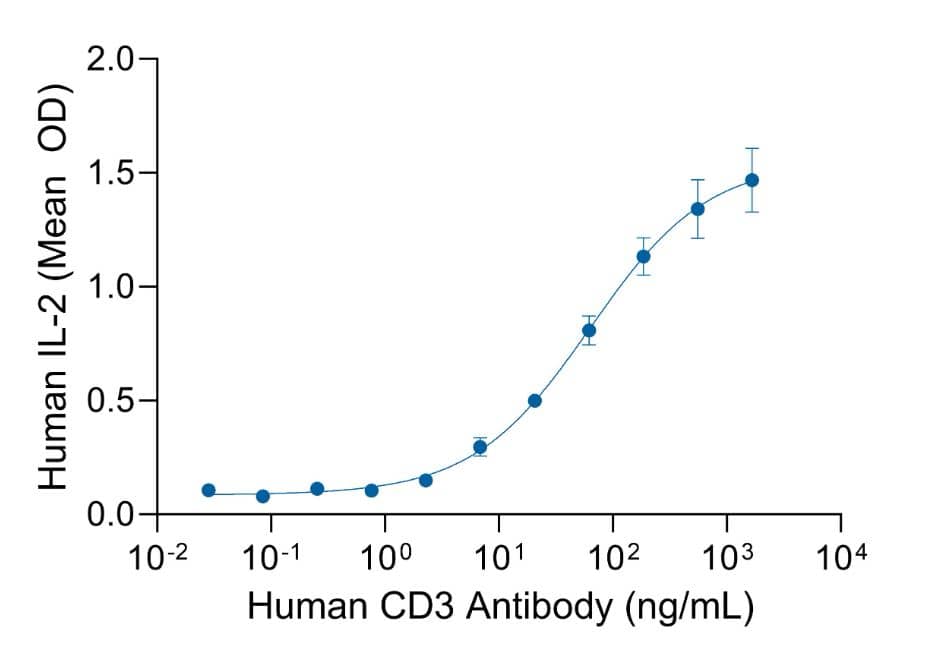
Recombinant Human CD3 GMP Antibody Stimulates IL-2 Secretion in Jurkat Cells.
Human CD3 GMP antibody stimulates IL-2 secretion in Jurkat cells (immortalized human T cell line) treated with 5 ng/mL phorbol myristate acetate (PMA). Antibody-mediated stimulation occurred in a dose-dependent manner, as measured using the Quantikine Human IL-2 ELISA kit. The ED50 for this effect is 8.00-80.0 ng/mL.Applications for Human CD3 GMP Antibody
Application
Recommended Usage
T Cell Stimulation
1 µg/mL
Sample: Human PBMCs
Sample: Human PBMCs
Formulation, Preparation, and Storage
Purification
Protein G purified from cell culture supernatant
Reconstitution
Reconstitute at 0.5 mg/mL in sterile PBS.
Formulation
Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. See Certificate of Analysis for details.
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below.
Stability & Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
- A minimum of 12 months when stored at ≤ -20 °C as supplied. Refer to lot specific COA for the Use by Date.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, ≤ -20 °C under sterile conditions after reconstitution.
Background: CD3
Alternate Names
CD_antigen: CD3e, CD3 antigen, delta subunit, CD3d antigen, CD3d antigen, delta polypeptide (TiT3 complex), CD3d molecule, delta (CD3-TCR complex), CD3-DELTA, CD3e, CD3e antigen, CD3e antigen, epsilon polypeptide (TiT3 complex), CD3e molecule, epsilon (CD3-TCR complex), CD3-epsilon, CD3G, CD3g antigen, CD3g antigen, gamma polypeptide (TiT3 complex), CD3g molecule, epsilon (CD3-TCR complex), CD3g molecule, gamma (CD3-TCR complex), CD3-GAMMA, FLJ17620, FLJ17664, FLJ18683, FLJ79544, FLJ94613, IMD18, MGC138597, T3DOKT3, delta chain, T3E, T-cell antigen receptor complex, epsilon subunit of T3, T-cell receptor T3 delta chain, T-cell surface antigen T3/Leu-4 epsilon chain, T-cell surface glycoprotein CD3 delta chain, T-cell surface glycoprotein CD3 epsilon chain, TCRE
Gene Symbol
CD3E
Additional CD3 Products
Product Documents for Human CD3 GMP Antibody
Manufacturing Specifications
GMP AntibodiesR&D Systems, a Bio-Techne Brand's GMP Antibodies are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products, and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented processes and QA control of documentation and process changes
- Personnel training programs
- Raw material inspection and supplier qualification/monitoring
- Fully validated equipment, processes and test methods
- Equipment calibration monitored and scheduled using a computerized calibration program
- Facility maintenance, safety programs and pest control
- Variances dispositioned using material review process
- Robust product stability program
R&D Systems strives to provide our customers with the analytical characteristics of each product so that customers may determine whether our products are appropriate for their research. The Certificate of Analysis provided contains the following lot specific information:
- Purity (SDS-PAGE)
- Aggregation (SEC)
- Endotoxin (LAL method, tested per USP<85> and Ph. Eur. 2.6.14 guidelines)
- Residual Host Cell Protein and Host Cell DNA
- Mycoplasma testing
- Post-bottling lot-specific bioassay results (compliance with an established range) and results of microbial testing according to USP <71>
Additional testing and documentation requested by the customer can be arranged at an additional cost.
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Product Specific Notices for Human CD3 GMP Antibody
Full terms and conditions of sale can be found online in the Protein Sciences Segment T&Cs at: Terms & Conditions.
For research use only
Loading...
Loading...
Loading...