
Abbreviations: iCE (imaged capillary electrophoresis), icIEF (imaged capillary isoelectric focusing), CE-SDS (capillary electrophoresis-sodium dodecyl sulfate)
Upgrading to new technologies is inevitable in the lab. Glass pipettes have evolved into digital ones for greater precision. Paper lab notebooks became electronic for better compliance and traceability. Manual gel electrophoresis was replaced by automated capillary systems for faster, more reproducible results. The same principle encourages the transition from the iCE3 to Maurice Systems for icIEF analysis: not just replacing an older instrument, but delivering higher-quality data with greater efficiency and confidence, along with a host of other benefits.
Maurice vs iCE3: Why Replace?
When the Maurice™ System was first released in 2016, it assured the following: faster icIEF analysis in just 10-15 minutes per sample, enhanced sensitivity with the option of native fluorescence detection (NF), and an additional analytical capability for measuring protein size and purity with CE-SDS. Designed to be a platform instrument for analytical needs, Maurice slowly began making its mark in the biopharmaceutical industry for its ease of use, robustness, reproducibility, and high-quality data.
The Maurice System’s predecessor is the iCE3 System, which has been widely adopted in the industry since 2012 for its unparalleled icIEF performance for measuring the charge heterogeneity in biotherapeutic molecules. However, significant improvements and additions led to the development of the Maurice system, which was also built on the same iCE technology. As Maurice increasingly started to be validated within the industry and widely implemented, the time came to retire the iCE3, which was ultimately discontinued in June 2022, with full instrument service and support available until June 30, 2029.
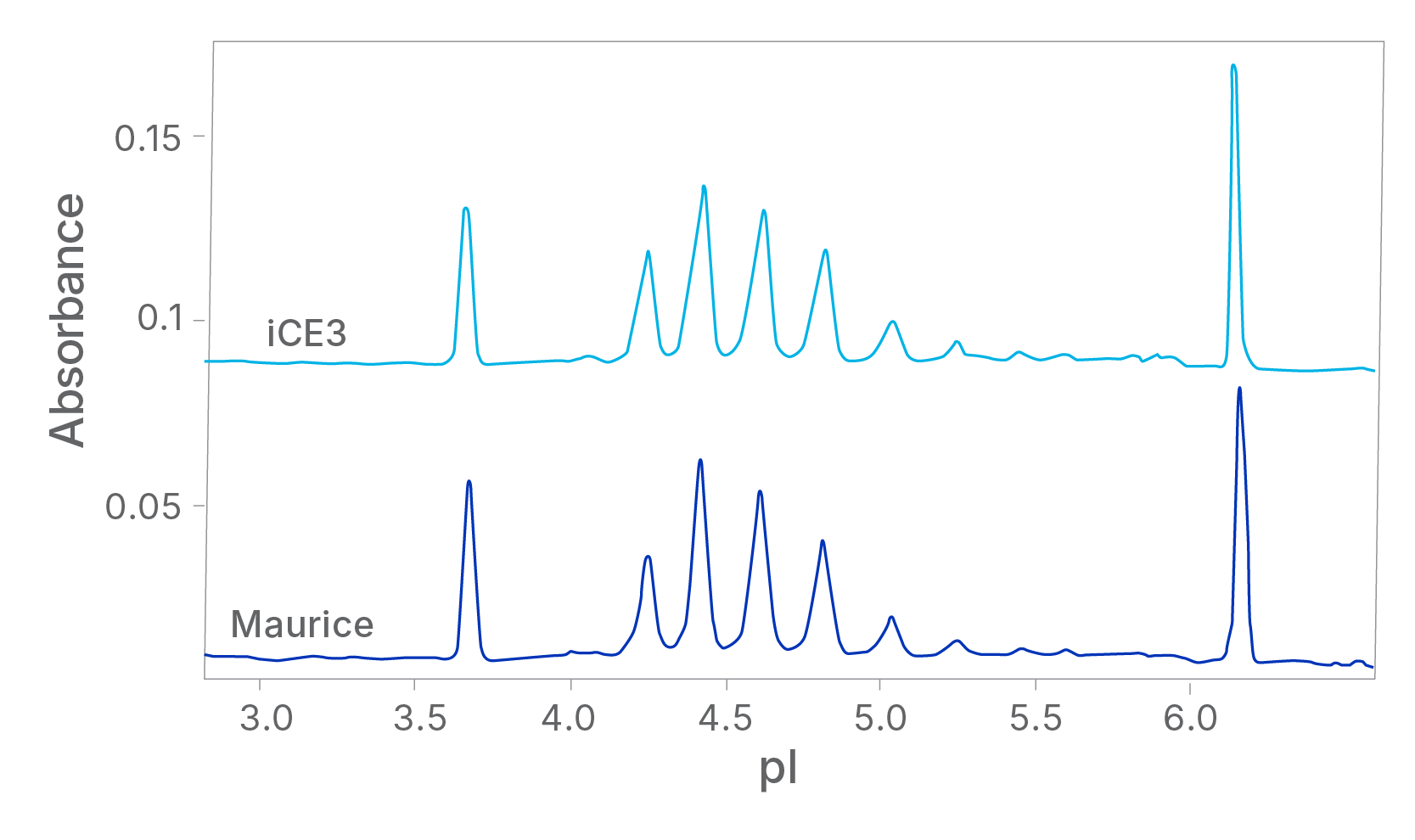
Proven Data Comparability between Maurice and iCE3
Understandably, the news of the iCE3 system’s discontinuation sparked some alarm. Even though Maurice was built on the same iCE technology, it became imperative to demonstrate data and performance comparability between both systems, considering that many commercial therapeutics were released using iCE3 assays. While internal data were generated that showed how comparable the two systems are, a large-scale global study involving 19 sites of different biopharmaceutical and IVD companies was implemented across North America, Europe, and China1,2.
Each site analyzed two molecules—a monoclonal antibody (NISTmAb) and a fusion protein (rhPD-L1-Fc) on both the iCE3 and Maurice. The results from this study were published in Electrophoresis in 2021 and stated “The iCE3 and Maurice instruments produce identical apparent pI values for the main isoform and comparable relative peak areas for the acidic, main, and basic isoforms for the NISTmAb. Both instruments also produce comparable quantitative results for rhPDL1-Fc”
Since the publication of the global multi-company study, several organizations have discussed their own experiences with transitioning from iCE3 to Maurice. Many have presented data at scientific talks, while some have published their findings through scientific posters or peer-reviewed papers. For example, a recent study published by Boehringer Ingelheim in Electrophoresis involved the comparison between iCE3 and Maurice C. performance in a QC environment, resulting in the conclusion that Maurice C. performed equal to or better than the iCE and satisfied all the acceptance criteria that were a part of the study design3. A poster presented by Lonza AG showed how the icIEF method on Maurice passed all acceptance criteria they had set while validating the method, concluding that the system was indeed fit for purpose for charge variant analysis4.
Why Switch Now?
Service and support for iCE3 are available until June 30th, 2029. While method transfer between iCE3 and Maurice is largely seamless, method validation and documentation can be time-consuming, therefore it is recommended to begin evaluating the Maurice system for your organization’s analytical requirements earlier, especially since several commercially released products and BLAs currently under review utilized iCE3 assays as a part of their regulatory filings. To facilitate the transition from iCE3 to Maurice, our team can support you with data from bridging studies and offers a trade-in program, where you can not only trade in your old iCE3 but also other instruments, such as the PA800 PLUS, GXII, and others (please inquire).
Continuous Innovation: MauriceFlex and Beyond
In 2023, the flagship MauriceFlex™ system was released, which does everything that Maurice does, but also enables offline fraction collection of charge isoforms. Having the ability to perform icIEF and icIEF-based fractionation on the same instrument eliminates the need to use other labor-intensive methods like ion exchange chromatography (IEX) and instead powers fast downstream analysis with intact and subunit mass spectrometric methods and even allows for binding potency analysis with surface plasmon resonance5,6,7.
Other efforts to increase lab productivity have led to innovations such as the Maurice icIEF 400 Cartridge, which enables twice the number of injections and batches per run compared to the standard Maurice cIEF Cartridge. Additionally, a faster icIEF method called SupersonicIEF, published in Electrophoresis in 2024, optimizes the standard Maurice icIEF protocol to deliver results two to three times faster while maintaining data quality and resolution8.
Maurice/MauriceFlex systems are of the same technology as the iCE3, and provide the same reliable results for icIEF, yet come with a plethora of benefits that will help to boost productivity and efficiency in the lab. If you’d like to learn more about upgrading to Maurice or MauriceFlex, download the iCE3 to Maurice Transition Support Brochure or contact your local Sales Representative.
References
- Application Note: iCE3 and Maurice Data Comparability Evaluated Using Three Biomolecules
- Madren, S., McElroy, W., Schultz-Kuszak, K., Boumajny, B., Shu, Y., Sautter, S., Zhao, H. C., Schadock-Hewitt, A., Chumsae, C., Ball, N., Zhang, X., Rish, K., Zhang, S., Wurm, C., Cai, S., Bauer, S. P., Stella, C., Zheng, L., Roper, B., Michels, D. A., … Mattila, M. (2022). Global intercompany assessment of ICIEF platform comparability for the characterization of therapeutic proteins. Electrophoresis, 43(9-10), 1050–1058. https://doi.org/10.1002/elps.202100348
- Ries, A. B., Merkel, M. N., Coßmann, K., Paul, M., Grunwald, R., Klemmer, D., Hübner, F., Eggensperger, S., & Weiß, F. T. (2025). Universal Study Design for Instrument Changes in Pharmaceutical Release Analytics. Electrophoresis, 10.1002/elps.70004. Advance online publication. https://doi.org/10.1002/elps.70004
- Scientific Poster: From iCE3 to Maurice: Qualification of the Maurice System and Implementation of the icIEF Test Method for mAb Charge Heterogeneity
- Application Note: Charge Variant Characterization of Innovator & Biosimilar Drugs with MauriceFlex & BioAccord LC-MS System
- Application Note: Comparing Charge Variants: Innovator vs Biosimilar Using the MauriceFlex System & Mass Spectrometry
- Application Note: A Novel icIEF Fractionation & SPR-Based Workflow for Correlating the Charge Structure to the Function of a Bispecific Antibody
- McElroy, W., & Heger, C. D. (2024). Development of the SupersonicIEF Method for High-Throughput Charge Variant Analysis. Electrophoresis, 45(21-22), 1968–1975. https://doi.org/10.1002/elps.202400117