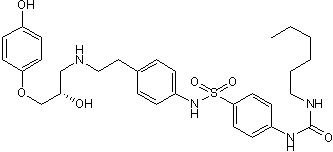
L-755,507
Tocris Bioscience, part of Bio-Techne | Catalog # 2197
Very potent and selective β3 partial agonist

Discontinued Product
2197 has been discontinued.
View all Adrenergic beta-3 Receptor Agonists products.
Key Product Details
| Description: | Very potent and selective β3 partial agonist |
| Chemical Name: | 4-[[(Hexylamino)carbonyl]amino]-N-[4-[2-[[(2S)-2-hydroxy-3-(4-hydroxyphenoxy)propyl]amino]ethyl]phenyl]-benzenesulfonamide |
| Purity: | ≥98% (HPLC) |
| Molecular Weight: | 584.73 |