Table of Contents
Multiplex Immunofluorescence (mIF) on COMET™
The COMET™ platform, from Lunaphore, a Bio-Techne brand, is a fully-automated, high-throughput, spatial biology platform that performs sequential immunofluorescence (seqIF™) through staining, imaging, and elution cycles. It offers the unique flexibility to work with off-the-shelf, non-conjugated primary antibodies.
Featured Multiplex IF Antibodies Qualified on COMET™
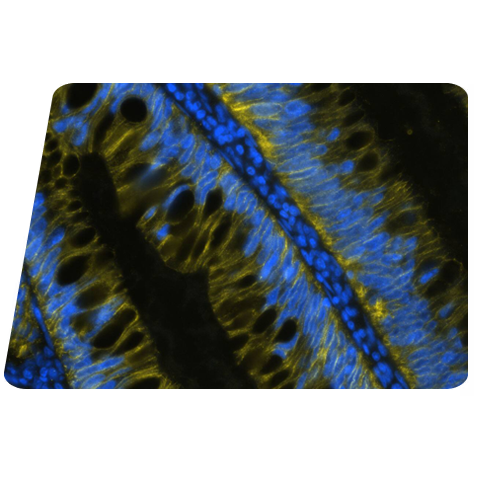
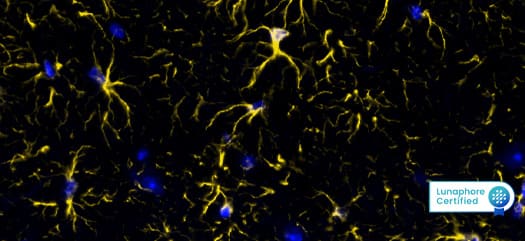
Figure 1: Detection of GFAP in Mouse Brain Cortex via seqIF™ staining on COMET™ using Rabbit Anti-Mouse GFAP Monoclonal Antibody (Catalog # NBP3-07877). Tissue was stained using the Alexa Fluor™ Plus 555 Goat anti-Rabbit IgG Secondary Antibody (Yellow; Lunaphore Catalog # DR555RB) and counterstained with DAPI (blue; Lunaphore Catalog # DR100). Specific staining was localized to the astrocytes showing cytoplasmic staining.
Figure 2: Detection of p53 in Human Colon Tumor via seqIF™ staining on COMET™ using Mouse Anti-Human p53 Monoclonal Antibody (Catalog # NBP2-29453). Tissue was stained using the Alexa Fluor™ 555 Goat anti-Mouse IgG Secondary Antibody (Yellow; Lunaphore Catalog # DR555MS) and counterstained with DAPI (blue; Lunaphore Catalog # DR100). Specific staining was localized to the astrocytes showing nuclear staining. Protocol available in COMET™ Panel Builder.
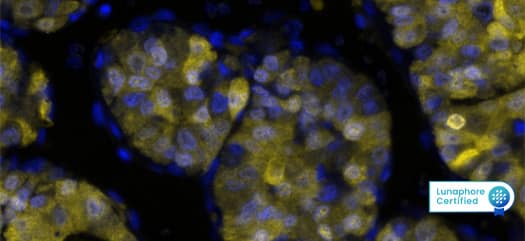
Figure 3: Detection of Arginase1 in Human Liver Tumor via seqIF™ staining on COMET™ using Mouse Anti-Human Arginase 1, Monoclonal Antibody (Catalog # MAB5868). Tissue was stained using the Alexa Fluor™ 647 Goat anti-Mouse IgG Secondary Antibody (Yellow; Lunaphore Catalog # DR647MS) and counterstained with DAPI (blue; Lunaphore Catalog # DR100). Specific staining was localized to the cytoplasm and nucleus.
COMET™ Qualified mIF Antibodies
Table 1: List of COMET™ Qualified mIF Antibodies from R&D Systems™ and Novus Biologicals™ part of Bio-Techne.
Antibody Testing Process on COMET™
Bio-Techne's antibody validation experts have collaborated and trained alongside Lunaphore's research and development team covering protocols and requirements involved in the antibody validation process on COMET™. This includes the antibody qualification process for protocol optimization, with a focus on assessing specificity, sensitivity, and elution efficiency using positive control tissue. At Bio-Techne, our IHC validated antibodies have already undergone a rigorous process to be added to the catalog. Antibody qualification process for COMET™ involves several key aspects as shown in Figure 1.
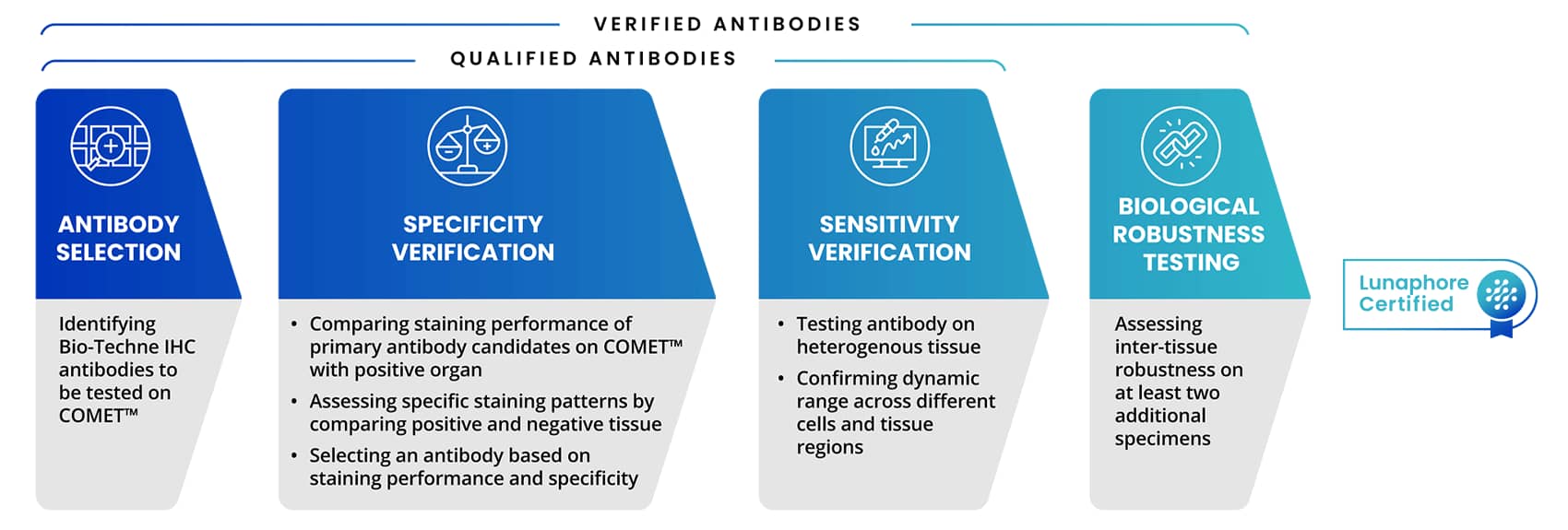
Figure 4. Steps of Antibody Testing Process on COMET™ that leads to Lunaphore Certified badge
Multiplex IF (mIF) validated antibodies with a Lunaphore certified badge are taken through the qualification or verification process listed above. This rigorous testing process ensures that antibodies, from R&D Systems™ and Novus Biologicals™, part of Bio-Techne, deliver high-quality and specific staining on the COMET™ platform. Bio-Techne Lunaphore Certified antibodies are also in the Lunaphore Panel Builder and added to the expanding COMET™ marker database.
Explore our curated selection of antibodies and take your spatial biology studies to new heights. All R&D Systems antibodies are provided in conjugation-ready format, free from BSA and Azide. Find our growing list of spatial biology tested antibodies from Novus Biologicals and R&D Systems below.
Have you used one of our antibodies in a spatial biology platform? Submit a review and receive a reward!
| Target | Platform | Catalog Number | External Data Link | OMAP_ID |
| ABCA3 | Cell DIVE™ | NBP1-89310 | Citation | OMAP-7 |
| AGER | Cell DIVE™ | AF1145 | Citation | OMAP-7 |
| APP / β Amyloid | Nanostring GeoMx® DSP | NBP2-13075AF647 | Data Image | |
| BST2 / CD317 | IMC (Hyperion™ and Hyperion Xti™) | DDX0390P-100 | Citation | |
| CD11b | Nanostring GeoMx® DSP | NB110-89474AF647 | Data Image | |
| CD161 / NK1.1 | IMC (Hyperion™ and Hyperion Xti™) | NB100-77528 | Citation | |
| CD20 | Nanostring GeoMx® DSP | NBP2-47840AF647 | Data Image | |
| CD20 | Nanostring GeoMx® DSP | NBP2-47840AF594 | Data Image | |
| CD20 | Nanostring GeoMx® DSP | NBP2-47840AF488 | Data Image | |
| CD3 | Nanostring GeoMx® DSP | NBP2-54392AF647 | Data Image | |
| CD3 | Nanostring GeoMx® DSP | NBP2-54392AF594 | Data Image | |
| CD3 | Nanostring GeoMx® DSP | NBP2-54392AF532 | Data Image | |
| CD3 | Nanostring GeoMx® DSP | NBP2-54392AF488 | Data Image | |
| CD4 | SIMS | NBP2-25199 | Citation | |
| CD4 | Canopy CellScape | FAB8165G | Citation | |
| CD68 | Nanostring GeoMx® DSP | NBP2-34587AF488 | Data Image | |
| CD68 | Nanostring GeoMx® DSP | NBP2-34587AF594 | Data Image | |
| CD68 | Nanostring GeoMx® DSP | NBP2-34587AF647 | Data Image | |
| CD68 | Nanostring GeoMx® DSP | NBP2-34587AF532 | Data Image | |
| CD69 | IMC (Hyperion™ and Hyperion Xti™) | NBP1-51607 | Data Image | |
| CD7 | IMC (Hyperion™ and Hyperion Xti™) | NBP2-37368 | Data Image | |
| CHGA | CODEX | NBP2-34674 | Citation 1, Citation 2 | OMAP-2, OMAP-6, OMAP-13 |
| C-Kit / CD117 | Nanostring GeoMx® DSP | NBP2-90037AF647 | Data Image | |
| CPS1 | SIMS | NBP3-08970 | Citation | OMAP-5 |
| CTSL | Cell DIVE™ | NB100-1775 | Citation | OMAP-7 |
| CXCL13 | IBEX | AF801 | Citation 1, Citation 2 | OMAP-1 |
| CXCR6 | IBEX | FAB2145R | Citation | |
| ELA2 / Neutrophil Elastase | Nanostring GeoMx® DSP | MAB9167AF594 | Data Image | |
| ELA2 / Neutrophil Elastase | Nanostring GeoMx® DSP | MAB9167AF488 | Data Image | |
| Fibronectin | IBEX | NBP2-22113AF532 | Citation | |
| GCG | CODEX | MAB12491 | Citation | OMAP-6 |
| Gfap | Nanostring GeoMx® DSP | NBP1-05197AF647 | Data Image | |
| Gfap | Nanostring GeoMx® DSP | NBP1-05197AF594 | Data Image | |
| Gfap | Nanostring GeoMx® DSP | NBP1-05197AF532 | Data Image | |
| Gfap | Nanostring GeoMx® DSP | NBP1-05197AF488 | Data Image | |
| Gfap | Nanostring GeoMx® DSP | NBP2-33184AF647 | Data Image | |
| Gfap | Nanostring GeoMx® DSP | NBP2-33184DL594 | Data Image | |
| Gfap | Nanostring GeoMx® DSP | NBP2-33184AF532 | Data Image | |
| Gfap | Nanostring GeoMx® DSP | NBP2-33184AF488 | Data Image | |
| Gfap | Nanostring GeoMx® DSP | NBP2-34413AF647 | Data Image | |
| GHRL | CODEX | MAB8200 | Citation | OMAP-13 |
| GP2 | CODEX | NBP2-75781 | Citation | OMAP-13 |
| HAVCR1 | CODEX | MAB1750 | Citation | OMAP-9 |
| HLA-G | IMC (Hyperion™ and Hyperion Xti™) | NB110-55297 | OMAP-8 | |
| ITLN1 | CODEX | AF4254 | Citation | OMAP-2 |
| KRT (Pan-Cytokeratin) | Nanostring GeoMx® DSP | NBP2-33200AF594 | Data Image | |
| KRT (Pan-Cytokeratin) | Nanostring GeoMx® DSP | NBP2-33200AF488 | Data Image | |
| KRT8 | CODEX | NBP2-34501 | Citation | OMAP-9 |
| KRT8/18 (Cytokeratin) | Nanostring GeoMx® DSP | NBP2-34655AF594 | Data Image | |
| Laminin (Pan-Laminin) | Nanostring GeoMx® DSP | NB300-144AF532 | Data Image | |
| Laminin (Pan-Laminin) | Nanostring GeoMx® DSP | NB300-144AF488 | Data Image | |
| Laminin (Pan-Laminin) | Nanostring GeoMx® DSP | NB300-144AF594 | Data Image | |
| Laminin (Pan-Laminin) | Nanostring GeoMx® DSP | NB300-144AF647 | Data Image | |
| Lgr5 | Nanostring GeoMx® DSP | NBP1-28904AF594 | Data Image | |
| LRP2 | CODEX | MAB9578 | Citation | OMAP-9 |
| LUM | IBEX | AF2846 | Citation 1, Citation 2 | OMAP-1, OMAP-11 |
| LYVE1 | IBEX, Cell DIVE™, IMC (Hyperion™ and Hyperion Xti™), CODEX | AF2089 | Citation 1, Citation 2, Citation 3, Citation 4, Citation 5 | OMAP-1, OMAP-4, OMAP-7, OMAP-8, OMAP-13 |
| MMR/CD206 | IMC (Hyperion™ and Hyperion Xti™) | AF2535 | Citation | |
| MRC1 | CODEX | AF2534 | Citation | OMAP-9 |
| MUC1 | Nanostring GeoMx® DSP | NBP2-47888AF594 | Data Image | |
| MUC2 | CODEX | NB120-11197 | Citation | OMAP-2 |
| Nefh | Nanostring GeoMx® DSP | NB500-416AF594 | Data Image | |
| NEFH | Nanostring GeoMx® DSP | NB500-416AF647 | Data Image | |
| Nefh | Nanostring GeoMx® DSP | NB500-416AF488 | Data Image | |
| Nefh | Nanostring GeoMx® DSP | NB500-416AF532 | Data Image | |
| NOS2 | Cell DIVE™ | NBP2-22119 | Citation | OMAP-7 |
| OCLN | Nanostring GeoMx® DSP | NBP3-08879AF594 | Data Image | |
| OCLN | Nanostring GeoMx® DSP | NBP3-08879AF647 | Data Image | |
| p53 | IBEX | NB200-103PE | Citation | |
| PECAM1 | IMC (Hyperion™ and Hyperion Xti™) | NB600-562 | OMAP-8 | |
| Perilipin | IMC (Hyperion™ and Hyperion Xti™) | NB110-40760 | Data Image | |
| PODXL | CODEX | AF1658 | Citation | OMAP-9 |
| PPY | CODEX | MAB62971 | Citation | OMAP-13 |
| SPARC | IBEX | AF941 | Citation 1, Citation 2 | OMAP-1, OMAP-11 |
| SST | CODEX | NBP2-37447 | Citation 1, Citation 2 | OMAP-6, OMAP-13 |
| SYP | CODEX | NBP1-47483 | Citation | OMAP-2 |
| TGF-beta 1 | IMC (Hyperion™ and Hyperion Xti™) | NBP1-80289 | Data Image | |
| UMOD | CODEX | AF5144 | Citation | OMAP-9 |
*NanoString and GeoMx are registered trademarks of NanoString Technologies, Inc.
*Hyperion™ and Hyperion Xti™ are registered trademarks of Standard BioTools.