Detection of Human MMR/CD206/Mannose Receptor by Immunocytochemistry/Immunofluorescence
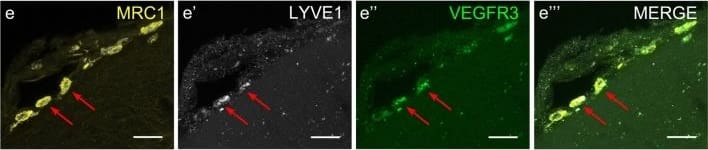
Cells of human meninges co-express LLEC markers. a–c DAB-IHC with single antibodies detects VEGFR3 (a), LYVE1 (b), and MRC1 (c) in the meninges of human post mortem brain showing no signs of neuropathology. These images are taken from a 38 year old male (sample P17/07, Table 1), and confirmed in n = 2 additional samples. P parenchyma. Scale = 150 µm (a); 40 µm (b); and 20 µm (c). d–f DAB-IHC with single antibodies detects VEGFR3 (b), LYVE1 (c), and MRC1 (d) in elderly human meninges (age: 89–92) with evidence of neuropathology and confirmed in n = 3 brains (Table 1). P, parenchyma. Scale = 20 µm. g–p IHC with fluorescent antibodies detects human meningeal cells that co-express MRC1 (h, m, yellow), LYVE1 (i, n, white), and VEGFR3 (j, o, green). Nuclei/RNA are labelled with DAPI (g, l, blue) and images are merged in (k, p). Scale = 10 µm Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31696318), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206/Mannose Receptor by Immunocytochemistry/Immunofluorescence
M1 and M2 phenotype in spinal cord after intraplantar IL-1 beta. Wild-type (WT) and LysM-G protein–coupled receptor kinase (GRK)2+/− mice received an intraplantar injection of 1 ng IL-1 beta. At 15 hours after injection, spinal cord was collected, and frozen sections of (A) lumbar spinal cord (L2 to L5) and as control (B) thoracic spinal cord (T6 to T10) were stained for M1 (CD16/32) and M2 (CD206 and arginase-I) phenotypic markers. A representative example of M1 and M2 staining in the dorsal horn of one of the four mice per group is displayed. Scale bar indicates 20 μm. (C) Quantification of microglia/macrophages expressing M1 and M2 phenotypic markers in spinal cord from WT and LysM-GRK2+/− mice. Expression was quantified in approximately 10 to 15 dorsal horns of spinal cords per group (4 mice per group). The level of expression in the lumbar or thoracic area from control WT mice was set at 100%. Data are expressed as means ± SEM. **P < 0.01, ***P < 0.001. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/22731384), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206/Mannose Receptor by Immunocytochemistry/Immunofluorescence
Immunofluorescent staining for macrophage marker F4/80 in Acomys and Mus.(A–C) Bone-marrow-derived cells isolated from Acomys and stained for F4/80 (green). (A) unstimulated cells, (B) cells stimulated with IFN gamma and LPS, (C) cells stimulated with IL-4. (D–F) Bone-marrow-derived cells isolated from Mus and stained for F4/80 (green). (D) unstimulated cells, (E) cells stimulated with IFN gamma and LPS, and (F) cells stimulated with IL-4. Scale bar = 50 μm. (G) Acomys ear tissue at D15 after injury stained for F4/80 (green), CD206 (red) and DAPI (grey). (H) Mus ear tissue at D7 after injury stained for F4/80 (green), CD206 (red) and DAPI (grey). Scale bar = 50 μm.DOI:https://dx.doi.org/10.7554/eLife.24623.012 Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28508748), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206/Mannose Receptor by Immunohistochemistry
Characterization of M2 BV2 cells induced by IL-4 and identification of sEVs derived from M2 BV2 cells. (A, B) Representative images of BV2 cells immunostained for Iba-1 (green), CD206 (red), and arginase (red). Cultured systems were treated with 0 or 20 ng/µL IL-4. Cell nuclei were counterstained with DAPI. Scale bar = 50 µm. (C) Western blotting analysis of CD206 and arginase expression in BV2 cells after 0 or 20 ng/µL IL-4 treatment. (D) Representative electron microscopy images showing the phenotype of M2-sEVs. Left image scale bar = 100 nm, right image scale bar = 50 nm. (F) NTA of M2-sEVs isolated by ultracentrifugation from M2 BV2 cells. Data represent the average size distribution profile of three samples and each purification normalized to the total nanoparticle concentrations. Data for each sample was derived from three different videos and analyses. (G) Western blotting analysis of TSG101 and CD63 levels in M2 BV2 cells and M2-sEVs. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33391532), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human MMR/CD206/Mannose Receptor by Immunocytochemistry/Immunofluorescence
Cells of human meninges co-express LLEC markers. a–c DAB-IHC with single antibodies detects VEGFR3 (a), LYVE1 (b), and MRC1 (c) in the meninges of human post mortem brain showing no signs of neuropathology. These images are taken from a 38 year old male (sample P17/07, Table 1), and confirmed in n = 2 additional samples. P parenchyma. Scale = 150 µm (a); 40 µm (b); and 20 µm (c). d–f DAB-IHC with single antibodies detects VEGFR3 (b), LYVE1 (c), and MRC1 (d) in elderly human meninges (age: 89–92) with evidence of neuropathology and confirmed in n = 3 brains (Table 1). P, parenchyma. Scale = 20 µm. g–p IHC with fluorescent antibodies detects human meningeal cells that co-express MRC1 (h, m, yellow), LYVE1 (i, n, white), and VEGFR3 (j, o, green). Nuclei/RNA are labelled with DAPI (g, l, blue) and images are merged in (k, p). Scale = 10 µm Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31696318), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206/Mannose Receptor by Immunocytochemistry/Immunofluorescence
Mouse LLECs take up A beta 1-40. a Schematic showing the site of dye and A beta1-40 perfusion into the CSF via the cisterna magna (arrow) of a 2-month old mouse. The dotted line indicates the plane of section. A anterior, P posterior, D dorsal, V ventral. b Coronal brain section indicating the areas imaged. SF4 refers to area captured in Figure S4. c The percentage of each labelled cell type that internalized perfused A beta. Cells co-expressing VEGFR3 and LYVE1 take up A beta at a higher rate than MRC1, LYVE1 double-positive cells as well as MRC1-positive, LYVE1-negative cells (p ≤ 0.05, bootstrap). VEGFR3, LYVE1 counts, n = 2 brains (3 sections/brain). MRC1, LYVE1 counts, n = 3 brains (3 sections/brain). d–d′′′ Cells of the adult mouse meninges that co-express VEGFR3 (d, green) and LYVE1 (d′, white) internalize A beta1-40 (d′′, cyan). Scale = 20 µm. e-e′′′) Cells of the adult mouse meninges that co-express VEGFR3 (e, green) and MRC1 (e′, white) internalize A beta1-40 (e′′, cyan). Scale = 40 µm. f–f′′′) Cells of the adult mouse meninges that co-express MRC1 (f, magenta) and LYVE1 (f′, white) internalize A beta1-40 (f′′, cyan). The walls of a blood vessel (white arrowhead, f′′) also accumulate A beta1-40. Scale = 60 µm Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31696318), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human MMR/CD206/Mannose Receptor by Immunocytochemistry/Immunofluorescence
Cells of human meninges co-express LLEC markers. a–c DAB-IHC with single antibodies detects VEGFR3 (a), LYVE1 (b), and MRC1 (c) in the meninges of human post mortem brain showing no signs of neuropathology. These images are taken from a 38 year old male (sample P17/07, Table 1), and confirmed in n = 2 additional samples. P parenchyma. Scale = 150 µm (a); 40 µm (b); and 20 µm (c). d–f DAB-IHC with single antibodies detects VEGFR3 (b), LYVE1 (c), and MRC1 (d) in elderly human meninges (age: 89–92) with evidence of neuropathology and confirmed in n = 3 brains (Table 1). P, parenchyma. Scale = 20 µm. g–p IHC with fluorescent antibodies detects human meningeal cells that co-express MRC1 (h, m, yellow), LYVE1 (i, n, white), and VEGFR3 (j, o, green). Nuclei/RNA are labelled with DAPI (g, l, blue) and images are merged in (k, p). Scale = 10 µm Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31696318), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206/Mannose Receptor by Immunocytochemistry/Immunofluorescence
Immunofluorescent staining for macrophage marker F4/80 in Acomys and Mus.(A–C) Bone-marrow-derived cells isolated from Acomys and stained for F4/80 (green). (A) unstimulated cells, (B) cells stimulated with IFN gamma and LPS, (C) cells stimulated with IL-4. (D–F) Bone-marrow-derived cells isolated from Mus and stained for F4/80 (green). (D) unstimulated cells, (E) cells stimulated with IFN gamma and LPS, and (F) cells stimulated with IL-4. Scale bar = 50 μm. (G) Acomys ear tissue at D15 after injury stained for F4/80 (green), CD206 (red) and DAPI (grey). (H) Mus ear tissue at D7 after injury stained for F4/80 (green), CD206 (red) and DAPI (grey). Scale bar = 50 μm.DOI:https://dx.doi.org/10.7554/eLife.24623.012 Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28508748), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206/Mannose Receptor by Immunocytochemistry/Immunofluorescence
Mouse LLECs take up A beta 1-40. a Schematic showing the site of dye and A beta1-40 perfusion into the CSF via the cisterna magna (arrow) of a 2-month old mouse. The dotted line indicates the plane of section. A anterior, P posterior, D dorsal, V ventral. b Coronal brain section indicating the areas imaged. SF4 refers to area captured in Figure S4. c The percentage of each labelled cell type that internalized perfused A beta. Cells co-expressing VEGFR3 and LYVE1 take up A beta at a higher rate than MRC1, LYVE1 double-positive cells as well as MRC1-positive, LYVE1-negative cells (p ≤ 0.05, bootstrap). VEGFR3, LYVE1 counts, n = 2 brains (3 sections/brain). MRC1, LYVE1 counts, n = 3 brains (3 sections/brain). d–d′′′ Cells of the adult mouse meninges that co-express VEGFR3 (d, green) and LYVE1 (d′, white) internalize A beta1-40 (d′′, cyan). Scale = 20 µm. e-e′′′) Cells of the adult mouse meninges that co-express VEGFR3 (e, green) and MRC1 (e′, white) internalize A beta1-40 (e′′, cyan). Scale = 40 µm. f–f′′′) Cells of the adult mouse meninges that co-express MRC1 (f, magenta) and LYVE1 (f′, white) internalize A beta1-40 (f′′, cyan). The walls of a blood vessel (white arrowhead, f′′) also accumulate A beta1-40. Scale = 60 µm Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31696318), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206/Mannose Receptor by Immunocytochemistry/Immunofluorescence
In vitro activation assays shows Acomys macrophages can be polarized to express different markers.(A–I) Bone-marrow-derived macrophages isolated from Acomys femurs are cultured with no cytokines (unstimulated, A, D, G) with IFN gamma+LPS (M1, B, E, H) or with IL-4 (M2, C, F, I). Immunocytochemistry for the pan-macrophage marker CD11b (green) (A–C), for the M1 macrophage marker CD86 (green) and the M2 macrophage marker Arginase 1 (red) (D–F), or CD206 (red) (G–I). (J–R). Bone-marrow-derived macrophages were isolated from Mus femurs and cultured with no cytokines (J, M, P) with IFN gamma and LPS (K, N, Q) or with IL4 (L, O, R) as above. Immunocytochemistry was performed for CD11b (green) (J–K), for CD86 (green) and Arginase 1 (red) (M–O), and CD206 (red) (P–R). Nuclei were counterstained with DAPI (grey) in all panels. Scale bars = 50 μm. Images are representative of n = 3 technical replicates.DOI:https://dx.doi.org/10.7554/eLife.24623.011Immunofluorescent staining for macrophage marker F4/80 in Acomys and Mus.(A–C) Bone-marrow-derived cells isolated from Acomys and stained for F4/80 (green). (A) unstimulated cells, (B) cells stimulated with IFN gamma and LPS, (C) cells stimulated with IL-4. (D–F) Bone-marrow-derived cells isolated from Mus and stained for F4/80 (green). (D) unstimulated cells, (E) cells stimulated with IFN gamma and LPS, and (F) cells stimulated with IL-4. Scale bar = 50 μm. (G) Acomys ear tissue at D15 after injury stained for F4/80 (green), CD206 (red) and DAPI (grey). (H) Mus ear tissue at D7 after injury stained for F4/80 (green), CD206 (red) and DAPI (grey). Scale bar = 50 μm.DOI:https://dx.doi.org/10.7554/eLife.24623.012 Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28508748), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206/Mannose Receptor by Immunohistochemistry
Microglial activation was attenuated by Hv1 deletion following LPC-induced demyelination. a Representative images of Iba-1 immunostaining in the CC of WT and Hv1−/− mice (scale bar, 200 μm). b Quantification of the number of microglia per high-power field (HPF) in the CC. Each point of WT and Hv1−/− mice, N = 5-7 mice. c Representative images of Iba-1 morphology and the corresponding 3D reconstructions (scale bars, magnified images, 20 μm; 3D reconstruction images, 5um) d Quantification analysis of the soma of microglia. Each point of WT and Hv1−/− mice, N = 4-6 mice, 6-15 cells per mouse. e Representative images of Iba-1 and CD16/32 co-localization in the CC of WT and Hv1−/− mice (scale bar, 50 μm; magnified images, 20 μm). f Quantification of the ratio of CD16/32+/Iba-1+. Each point of WT mice, N = 5-8 mice; Hv1−/− mice, N = 5-8 mice. g Representative images of Iba-1 and CD206 co-localization in the CC of WT and Hv1−/− mice (scale bar, 50 μm; magnified images, 20 μm). h Quantification of the ratio of CD206+/Iba-1+. Each point of WT mice, N = 6-7 mice; Hv1−/− mice, N = 5-6 mice. Data are shown as mean ± SD, *P < 0.05, ***P<0.001, two-way ANOVA with Dunnett’s post hoc test Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33158440), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206/Mannose Receptor by Immunocytochemistry/Immunofluorescence
Cells with BLEC molecular markers are present within the mouse leptomeninges. a Coronal brain section of adult zebrafish brain indicating the imaging area in the dorsal optic tectum (TeO). b A 14 month old Tg(kdr-l:mCherry); Tg(flt4:mCitrine) double transgenic zebrafish has cells in the meninges (white bracket) that express flt4/vegfr3 ( alpha-GFP, green) near kdr-l positive ( alpha-RFP, red) blood vessels. DAPI (blue) labels the nuclei. Scale = 50 µm. c Coronal mouse brain section showing the imaging areas of the meninges. d As revealed by IHC, 17-week-old mouse brains express VEGFR3 (green) in the meninges (white bracket). Tie2-GFP;NG2-DsRed double reporter mice were used to distinguish arteries and veins. NG2 (red) labels pericytes and smooth muscle cells, Tie2 (magenta) labels vascular endothelial cells, and Hoechst (blue) stains nuclei. The image is rotated with the parenchyma at the bottom for ease of comparison with panel b. Scale = 50 µm. e-e′′′ As revealed by IHC, cells of the meninges co-express MRC1 (e, yellow), LYVE1 (e′, white), and VEGFR3 (e′′, green). Red arrows highlight cells expressing these three markers. The images are rotated with the parenchyma at the bottom. scale = 30 µm. f, g Quantification of the relative numbers of single and double-labelled cells in 2-month old mouse meninges. VEGFR3 and LYVE1 cell counts were from n = 2 brains, 3 coronal sections (10 area images)/brain. MRC1 and LYVE1 cell counts were from n = 3 brains, 3 coronal sections (4 area images)/brain. The mean values for each set are depicted Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31696318), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206/Mannose Receptor by Immunocytochemistry/Immunofluorescence
M1 and M2 phenotype in spinal cord after intraplantar IL-1 beta. Wild-type (WT) and LysM-G protein–coupled receptor kinase (GRK)2+/− mice received an intraplantar injection of 1 ng IL-1 beta. At 15 hours after injection, spinal cord was collected, and frozen sections of (A) lumbar spinal cord (L2 to L5) and as control (B) thoracic spinal cord (T6 to T10) were stained for M1 (CD16/32) and M2 (CD206 and arginase-I) phenotypic markers. A representative example of M1 and M2 staining in the dorsal horn of one of the four mice per group is displayed. Scale bar indicates 20 μm. (C) Quantification of microglia/macrophages expressing M1 and M2 phenotypic markers in spinal cord from WT and LysM-GRK2+/− mice. Expression was quantified in approximately 10 to 15 dorsal horns of spinal cords per group (4 mice per group). The level of expression in the lumbar or thoracic area from control WT mice was set at 100%. Data are expressed as means ± SEM. **P < 0.01, ***P < 0.001. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/22731384), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human MMR/CD206/Mannose Receptor by Immunocytochemistry/Immunofluorescence
Cells of human meninges co-express LLEC markers. a–c DAB-IHC with single antibodies detects VEGFR3 (a), LYVE1 (b), and MRC1 (c) in the meninges of human post mortem brain showing no signs of neuropathology. These images are taken from a 38 year old male (sample P17/07, Table 1), and confirmed in n = 2 additional samples. P parenchyma. Scale = 150 µm (a); 40 µm (b); and 20 µm (c). d–f DAB-IHC with single antibodies detects VEGFR3 (b), LYVE1 (c), and MRC1 (d) in elderly human meninges (age: 89–92) with evidence of neuropathology and confirmed in n = 3 brains (Table 1). P, parenchyma. Scale = 20 µm. g–p IHC with fluorescent antibodies detects human meningeal cells that co-express MRC1 (h, m, yellow), LYVE1 (i, n, white), and VEGFR3 (j, o, green). Nuclei/RNA are labelled with DAPI (g, l, blue) and images are merged in (k, p). Scale = 10 µm Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31696318), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206/Mannose Receptor by Immunohistochemistry
Representative images of HE, M3/84 (macrophages) and MR (CD206) immunohistochemistry. In control mice, no plaques with macrophages were observed, while fibrous/fibroatheromatous plaques were present in the aortas extracted from ApoE-KO mice. The lesions (fatty streaks and fibrous plaques) showed high amounts of MR+ macrophages (100 μm (bars), vascular lumen (L), intima (arrow), media (asterisk), adventitia (arrowhead)) Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28470406), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Mouse MMR/CD206 Antibody by Immunohistochemistry
TAMs along the beam paths show a mixed M2-like/M1-like phenotype and expression of phagocytosis markers. (A) Double staining for CD68 (red) and CD206 (green) plus DAPI (blue) shows at 7 days post-MRT that many of the underlined TAMs are positive for both markers, indicating their inclination towards an M2-like phenotype. Dashed white lines demarcate the border between the tumour (lower part) and the normal tissue (upper part). (B) Triple staining for CD68 (red), CD206 (green) and Dectin-1 (grey) shows an abundant triple positive TAM population along the beam paths, indicating their inclination towards a phagocytic phenotype. (C) Double staining for CD68 (red) and Ly6C (cyan) reveals a partial M1-like TAM population and, at the same time, reveals cells that are not double positive, indicating the presence of recruited monocytes. Dashed yellow lines indicate a macrophage cluster along a microbeam path. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35453485), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206/Mannose Receptor by Immunohistochemistry
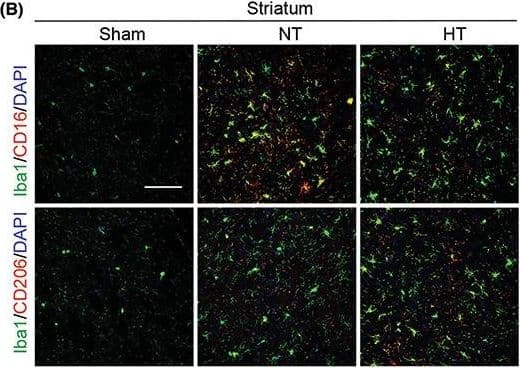
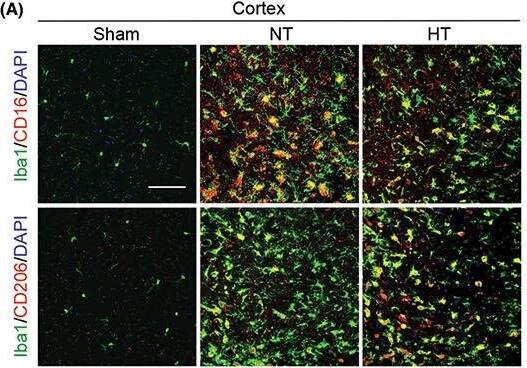
Hypothermia promotes a shift of microglia/macrophages towards an anti‐inflammatory phenotype in aged female mice 7 days after ischemic stroke. Mice were subjected to distal MCAO (dMCAO) or sham surgery. Normothermia (NT) or hypothermia (HT) was induced for 50 min immediately after dMCAO. Coronal brain sections were stained for Iba‐1 (a microglial/macrophage marker) and CD206 (an anti‐inflammatory marker) or CD16 (a pro‐inflammatory marker). (A) Representative images of Iba1/CD16 and Iba1/CD206 immunofluorescence staining in the ipsilateral peri‐infarct cortex (CTX) regions. (B) Representative images showing Iba1/CD16 and Iba1/CD206 immunofluorescence staining in the ipsilateral peri‐infarct striatum (STR) regions. (C) Representative magnified 3‐dimensional images of Iba1/CD16 and Iba1/CD206 staining. (D) Quantification of the total number of Iba1+ cells (upper panel), Iba1+/CD16+ cells (middle panel), and Iba1+/CD206+ cells (lower panel). The number of double‐positive cells was expressed as the number over 100 Iba1+ cells. n = 5 for sham (S), n = 6 for NT and n = 6 for HT. (E) Pearson correlation analysis of NeuN+ cells with Iba1+/CD206+ cells in the CTX region, n = 6 per group. (F) Pearson correlation analysis of beta‐APP+ cells with Iba1+/CD206+ cells in the CTX region, n = 6 per group. Scale bar: 50 μm. Data are shown as mean ± SD. *p < 0.05, **p < 0.01 NT vs. HT, ns, no significance. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36341958), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206/Mannose Receptor by Western Blot
HCK knockout reduced kidney fibrosis in uIRIx model with regulation of macrophage activation.A Schematic diagram of strategy of uIRIx models. B Urine ACR and serum BUN for WT and HCK KO uIRIx mice. C Representative images of H&E, COL1A1 IF and Masson trichrome staining from WT and KO uIRIx kidneys. Morphometric quantification (n = 5 animals; 5 random fields/animal) of the COL1A1 (top) and Masson (lower) positive area. D Representative IF staining images and quantification of from kidneys of HCK and F4/80 macrophage from WT and HCK KO uIRIx kidneys. E Western blot and quantification of M1 and M2 macrophage markers from WT and HCK KO mice kidneys at 28 days post uIRIx. F Representative IF staining images and quantification of LC3 in macrophages from WT & HCK KO uIRIx kidneys. B, C and E*p < 0.05 with t test. Source data are provided as a Source Data file. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37463911), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206/Mannose Receptor by Immunohistochemistry
Hypothermia promotes a shift of microglia/macrophages towards an anti‐inflammatory phenotype in aged female mice 7 days after ischemic stroke. Mice were subjected to distal MCAO (dMCAO) or sham surgery. Normothermia (NT) or hypothermia (HT) was induced for 50 min immediately after dMCAO. Coronal brain sections were stained for Iba‐1 (a microglial/macrophage marker) and CD206 (an anti‐inflammatory marker) or CD16 (a pro‐inflammatory marker). (A) Representative images of Iba1/CD16 and Iba1/CD206 immunofluorescence staining in the ipsilateral peri‐infarct cortex (CTX) regions. (B) Representative images showing Iba1/CD16 and Iba1/CD206 immunofluorescence staining in the ipsilateral peri‐infarct striatum (STR) regions. (C) Representative magnified 3‐dimensional images of Iba1/CD16 and Iba1/CD206 staining. (D) Quantification of the total number of Iba1+ cells (upper panel), Iba1+/CD16+ cells (middle panel), and Iba1+/CD206+ cells (lower panel). The number of double‐positive cells was expressed as the number over 100 Iba1+ cells. n = 5 for sham (S), n = 6 for NT and n = 6 for HT. (E) Pearson correlation analysis of NeuN+ cells with Iba1+/CD206+ cells in the CTX region, n = 6 per group. (F) Pearson correlation analysis of beta‐APP+ cells with Iba1+/CD206+ cells in the CTX region, n = 6 per group. Scale bar: 50 μm. Data are shown as mean ± SD. *p < 0.05, **p < 0.01 NT vs. HT, ns, no significance. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36341958), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of MMR/CD206 by Immunohistochemistry
Quantification of the number of perivascular macrophages (PVMs). The number of PVMs in the cerebral cortex was counted and compared between the control and CB-NP exposure groups. (A–F) Low-magnification images to quantify the number of PVMs using MMR expression. (A,D) are merged pictures of (B,C,E,F), respectively. Arrowheads indicate MMR-positive PVMs. (G) Number of PVMs in the cerebral cortex. Values are expressed as the mean ± SD. Abbreviation: Carbon black nanoparticle (CB-NP), Cerebral cortex (Cx), Corpus callosum (CC), and Striatum (Str), Standard deviation (SD). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35391824), licensed under a CC-BY license. Not internally tested by R&D Systems.
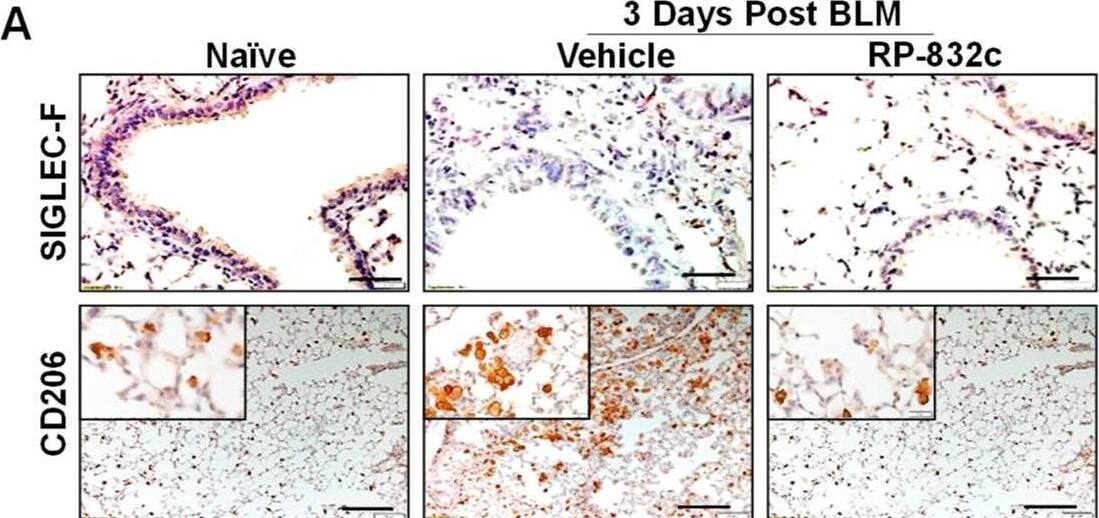
Detection of MMR/CD206 by Immunohistochemistry
RP-832c peptide levels significantly decreased in CD206 + macrophages and the profibrotic markers alpha-SMA and TGF-beta 1 without affecting Siglec-F-positive alveolar macrophages. (A) Representative 20× and 40× images of immunohistochemistry staining using anti-Siglec-F and anti-CD206 antibodies in lung tissues of naïve mice, as well as BLM-challenged vehicle/RP-832c-treated mice. The calibration bar represents 10 µm. (B) Representative 20× and 40× images of immunofluorescence staining of lung tissues using antibodies for alpha-SMA (red) and TGF beta-1 (green). The calibration bar represents 20 µm. (C–F) Bar graphs showing the quantifications of the IHC (Siglec-F & CD206) and IF ( alpha-SMA & TGF-beta 1) staining of lung tissues. Quantifications was done using Metamorph imaging software. S. E. **** p < 0.0001, *** p < 0.001, ** p < 0.01 and * p < 0.05 were considered significant. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37174654), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of MMR/CD206 by Immunohistochemistry
RP-832c peptide levels significantly decreased in CD206 + macrophages and the profibrotic markers alpha-SMA and TGF-beta 1 without affecting Siglec-F-positive alveolar macrophages. (A) Representative 20× and 40× images of immunohistochemistry staining using anti-Siglec-F and anti-CD206 antibodies in lung tissues of naïve mice, as well as BLM-challenged vehicle/RP-832c-treated mice. The calibration bar represents 10 µm. (B) Representative 20× and 40× images of immunofluorescence staining of lung tissues using antibodies for alpha-SMA (red) and TGF beta-1 (green). The calibration bar represents 20 µm. (C–F) Bar graphs showing the quantifications of the IHC (Siglec-F & CD206) and IF ( alpha-SMA & TGF-beta 1) staining of lung tissues. Quantifications was done using Metamorph imaging software. S. E. **** p < 0.0001, *** p < 0.001, ** p < 0.01 and * p < 0.05 were considered significant. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37174654), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of MMR/CD206 by Immunohistochemistry
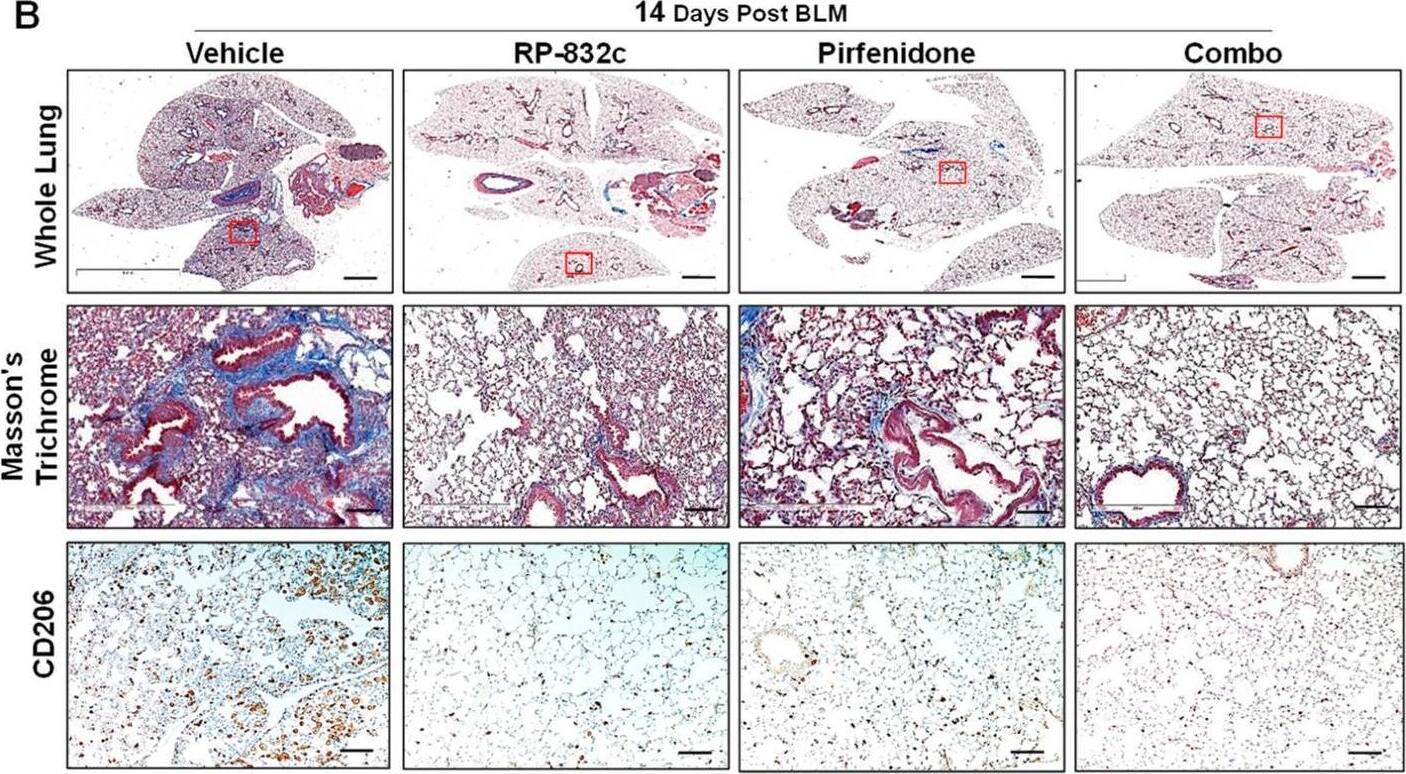
RP-832c peptide is comparable to Pirfenidone in decreasing fibrosis in an established late model of BLM-induced lung fibrosis. (A) A schematic of the animal study in which mice were challenged with a 2.5 U/kg body weight dose of BLM at day 0. A total of 14 days post-BLM challenge, mice were treated with either a vehicle, 10 mg/kg RP-832c (QD), 30 mg/kg Pirfenidone (Q3D), or a combination of RP-832c and Pirfenidone for an additional 21 days. (B) Histological analysis of lung tissues. The upper panel shows representative 2× images of Masson’s Trichrome-stained whole lung tissue, the middle panel contains representative 20× images, and the lower panel shows 10× images representing anti-CD206 immunohistochemical staining of lung tissue. (C) Modified Ashcroft scoring of each treatment group, determined after 21 days of treatment. n = 6 per treatment group. S. E. ** p < 0.001, and * p < 0.05 were considered significant. (D) Quantification of the anti-CD206 IHC-stained lung tissues. The bar represents 10 µm. n = 6 per treatment group. S. E. **** p < 0.0001, ** p < 0.001, and * p < 0.05 were considered significant. (E) Body weights of the mice measured over the course of treatment in each group. n = 6 per treatment group. (F) Lung weights of the mice measured at the end of the study. n = 6 per treatment group. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37174654), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of MMR/CD206 by Immunohistochemistry
RP-832c peptide is comparable to Pirfenidone in decreasing fibrosis in an established late model of BLM-induced lung fibrosis. (A) A schematic of the animal study in which mice were challenged with a 2.5 U/kg body weight dose of BLM at day 0. A total of 14 days post-BLM challenge, mice were treated with either a vehicle, 10 mg/kg RP-832c (QD), 30 mg/kg Pirfenidone (Q3D), or a combination of RP-832c and Pirfenidone for an additional 21 days. (B) Histological analysis of lung tissues. The upper panel shows representative 2× images of Masson’s Trichrome-stained whole lung tissue, the middle panel contains representative 20× images, and the lower panel shows 10× images representing anti-CD206 immunohistochemical staining of lung tissue. (C) Modified Ashcroft scoring of each treatment group, determined after 21 days of treatment. n = 6 per treatment group. S. E. ** p < 0.001, and * p < 0.05 were considered significant. (D) Quantification of the anti-CD206 IHC-stained lung tissues. The bar represents 10 µm. n = 6 per treatment group. S. E. **** p < 0.0001, ** p < 0.001, and * p < 0.05 were considered significant. (E) Body weights of the mice measured over the course of treatment in each group. n = 6 per treatment group. (F) Lung weights of the mice measured at the end of the study. n = 6 per treatment group. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37174654), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of MMR/CD206 by Western Blot
DSS-treated TLR4-SNP mice exhibit reduced M2a markers in the colon and reduced beta-hydroxybutyrate ( beta-HB) levels in the sera. (A) M2a gene expression measured in the MC of male WT vs TLR4-SNP mice, comparing control, Day 9, and Day 11 samples by qRT-PCR. One-way ANOVA with Sidak multiple comparisons test. *P < 0.05; **P < 0.01; ****P < 0.0001. Data are derived from two independent experiments. In total, control WT n = 11; control TLR4-SNP n = 15; WT Day 9 n = 13, Day 11 n = 13; TLR4-SNP Day 9 n = 15, Day 11 n = 15. (B) M2a (Ym1, Arg1) protein production measured in DC samples in WT vs TLR4-SNP mice, comparing control and Day 11 samples. Data are representative of results from two independent experiments totaling control WT n = 5; control TLR4-SNP n = 5; WT Day 11 n = 9; TLR4-SNP Day 11 n = 9. (C) M2a (Mrc1, PPAR gamma) protein production measured in DC samples in WT vs TLR4-SNP mice, comparing control and Day 11 samples. Data representative of results from two independent experiments totaling control WT n = 5; control TLR4-SNP n = 5; WT Day 11 n = 9; TLR4-SNP Day 11 n = 9. (D) beta-HB concentration measured in the sera of control and DSS-treated, WT vs TLR4-SNP male mice at Days 9 and 11. Two-way ANOVA with Sidak multiple comparisons. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Data are derived from three independent experiments in which, together, control WT n = 12; control TLR4-SNP n = 13; WT Day 11 n = 10; TLR4-SNP Day 11 n = 10. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37768050), licensed under a CC-BY license. Not internally tested by R&D Systems.
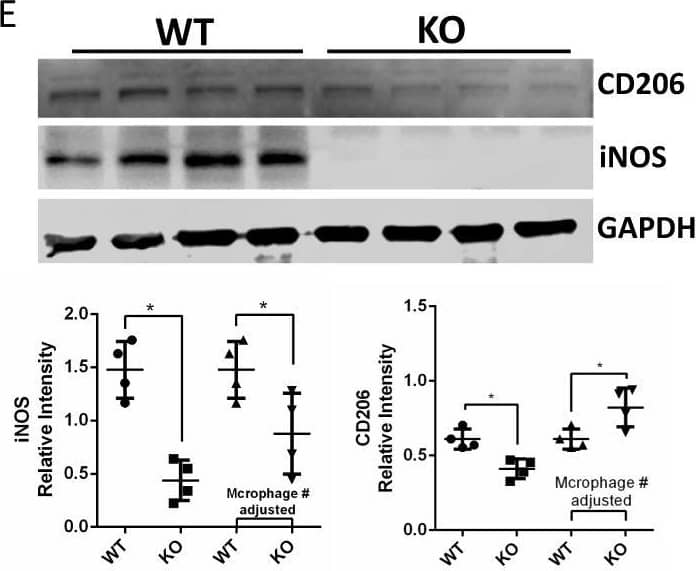
Detection of Mouse MMR/CD206 by Western Blot
In vivo experiment of TA gel in the mouse a the protocol of in vivo experiment; b in vivo fluorescence images of the TA gel at different implantation time points; c representative WB stripes of iNOS and CD206 expressions in the stroke mice; quantitative WB results of d iNOS and e CD206; ethological results of f balance beam and g rotarod tests Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37908012), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206 by Western Blot
In vitro cell culture a schematic diagram of BV2 cell culture; b BV2 cell viabilities treated with different TAG dilutions before and after OGD; c cytotoxicity by LDH measurement before and after OGD; d IF images of CD16 and CD206 staining in BV2 cells after OGD; semiquantitative results of e CD16 and f CD206 staining; g representative WB strips of CD16, IL-1 beta, CD206 and TGF-beta expression in microglia before and after OGD; quantitative WB results of h CD16, i IL-1 beta, j CD206 and k TGF-beta ; l N2a cell viabilities cocultured with BV2 cells before and after OGD; m schematic diagram of N2a and BV2 cell coculture; n representative WB stripes of synaptophysin and PSD95 expression in N2a coculture with BV2 cells before and after OGD; quantitative WB results of o synaptophysin and p PSD95 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37908012), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse MMR/CD206 by Immunocytochemistry/ Immunofluorescence
Effect of human umbilical cord mesenchymal stem cell‐derived exosomes on the microglia cell polarization. (A and B) Western blot analysis and quantification of iNOS and COX‐2. (C and D) Western blot analysis and quantification of YM1 and ARG1. (E) Representative images of microglia bearing a pro‐inflammatory (M1) phenotype in each group were examined using anti‐CD86 (green) and anti‐Iba‐1 (red) antibodies, as well as DAPI (blue); scale bar = 25 μm. (F) Representative images of anti‐inflammatory phenotype microglia in each group were examined using anti‐CD206 (green), and anti‐Iba‐1 (red) antibodies, as well as DAPI (blue); scale bar = 25 μm. (G) Relative fluorescence intensity analysis for CD86 and CD206. (H) Representative images of primary microglia bearing a pro‐inflammatory phenotype in each group were examined using anti‐CD86 (green), anti‐Iba‐1 (red) antibodies, and DAPI (blue); scale bar = 25 μm. (I) Representative images of anti‐inflammatory phenotype primary microglia in each group were examined by using anti‐CD206 (green) and anti‐Iba‐1 (red) antibodies, as well as DAPI (blue); scale bar = 25 μm. (J) Relative fluorescence intensity analysis for CD86 and CD206 in primary microglia; n = 3; mean ± SD; *p < 0.05; **p < 0.01; ***p < 0.001, compared with the control group; #p < 0.05; ##p < 0.01; ###p < 0.001, compared with the LPS group. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37697971), licensed under a CC-BY license. Not internally tested by R&D Systems.