Recombinant Human IL-15 GMP Protein, CF GMP Best Seller
R&D Systems, part of Bio-Techne | Catalog # BT-015-GMP
Animal-Free


New! Bypass reconstitution steps by using a liquid formulation of GMP-grade Recombinant Human IL-15. Find out more here.
Key Product Details
- Single use vials supplement 1L of T Cell Media (Cat # CCM038-GMP) to support T Cell Cultures in a G-Rex 100M bioreactor
- Liquid formulation minimizes reconstitution steps
- Lot-to-lot consistency
- Stringent guidelines for patient safety
- Scalability necessary to support successful therapeutics
- Learn more about manufacturing in our new GMP facility
- Test it in your process! Request a sample of GMP IL-15
Product Specifications
Source
E. coli-derived human IL-15 protein
Asn49-Ser162
Produced using non-animal reagents in an animal-free laboratory.
Manufactured and tested under cGMP guidelines.
Asn49-Ser162
Produced using non-animal reagents in an animal-free laboratory.
Manufactured and tested under cGMP guidelines.
Purity
>97%, by SDS-PAGE with quantitative densitometry by Coomassie® Blue Staining. The molecular weight by mass spectrometry is 12761
Da ± 5 Da.
Endotoxin Level
<0.10 EU per 1 μg of the protein by the LAL method.
N-terminal Sequence Analysis
Asn49-Trp-Val-Asn-Val-Ile-Ser-Asp-Leu-Lys
Predicted Molecular Mass
13 kDa
SDS-PAGE
9 kDa, under reducing conditions
Activity
Measured in a cell proliferation assay using MO7e human megakaryocytic leukemic cells.
The ED50 for this effect is 0.300-2.60 ng/mL. The specific activity of recombinant human IL-15 is >2.00 x 108 units/mg, which is calibrated against the human IL-15 reference standard (NIBSC code: 95/554).
The ED50 for this effect is 0.300-2.60 ng/mL. The specific activity of recombinant human IL-15 is >2.00 x 108 units/mg, which is calibrated against the human IL-15 reference standard (NIBSC code: 95/554).
Host Cell Protein
<1.00 ng per μg of protein when tested by ELISA.
Mycoplasma
Negative for Mycoplasma.
Host Cell DNA
<0.0100 ng per µg of protein when tested by PCR.
Scientific Data Images for Recombinant Human IL-15 GMP Protein, CF
Recombinant Human IL-15 Protein GMP Bioactivity.
GMP-grade Recombinant Human IL-15 (Catalog # BT-015-GMP) stimulates cell proliferation in the MO7e human megakaryocytic leukemic cell line. The ED50for this effect is 0.300-2.60 ng/mL. Three independent lots were tested for activity and plotted on the same graph to show lot-to-lot consistency of GMP IL-15.Recombinant Human IL-15 GMP Protein SDS-PAGE
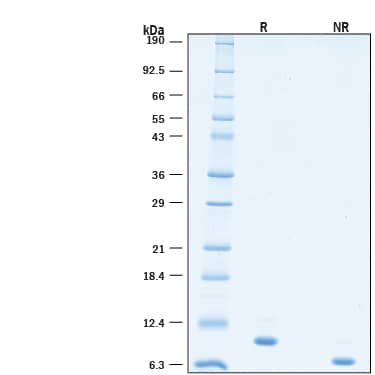
2 μg/lane of GMP-grade Recombinant Human IL-15 (Catalog # BT-015-GMP) was resolved with SDS-PAGE under reducing (R) conditions and visualized by Coomassie® Blue staining, showing a single band at 9 kDa.Recombinant Human IL-15 GMP Protein Measured by Quantikine ELISA.
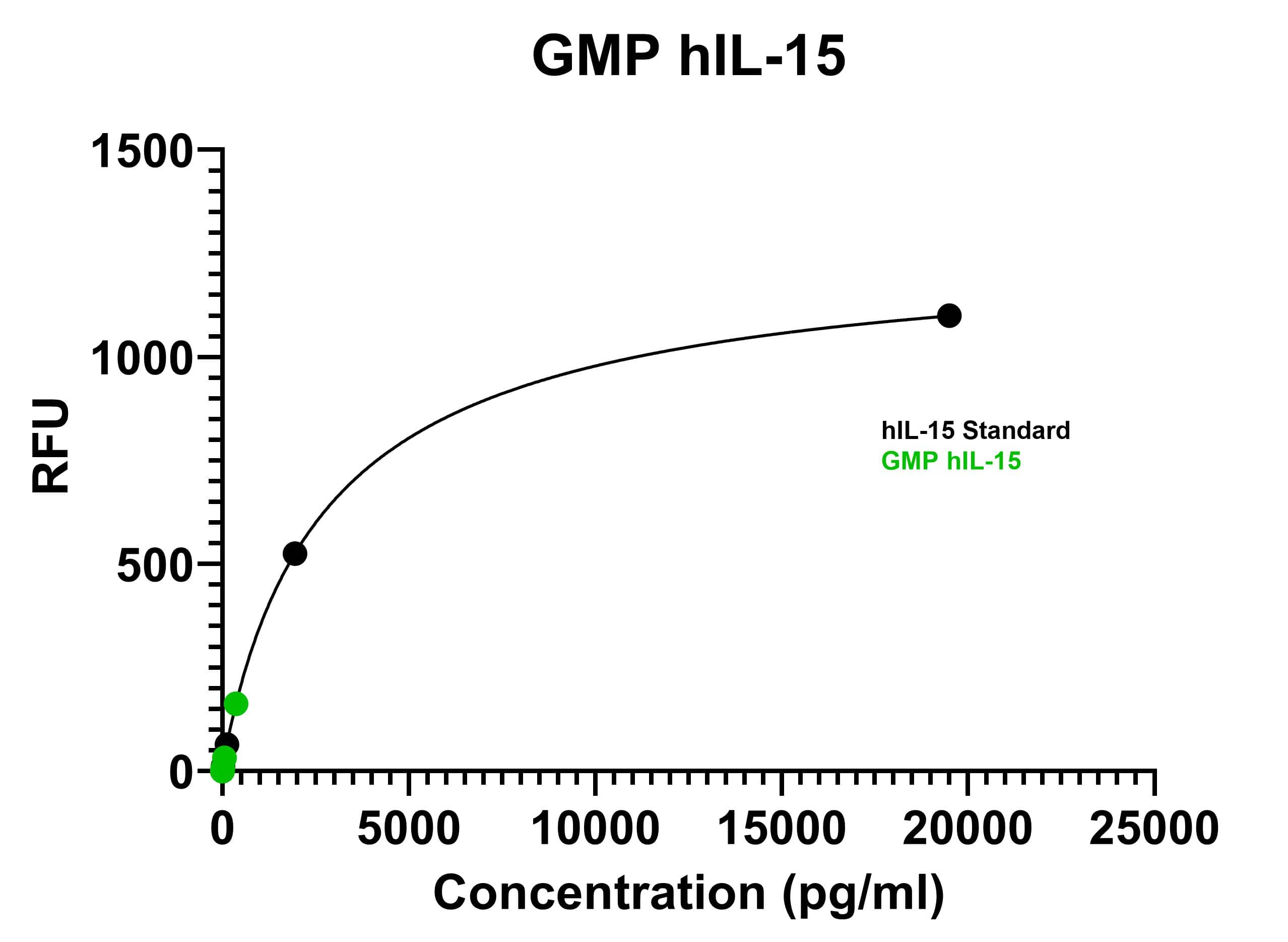
Two samples of GMP-grade human IL-15 (Catalog number BT-015-GMP, green circles) were interpolated with the human IL-15 Quantikine ELISA (D1500) standard curve (black circles) using 4PL regression analysis. The human IL-15 Quantikine ELISA has an assay range of 3.9-250 pg/mL.Formulation, Preparation and Storage
Lyophilized: BT-015-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 100-500 μg/mL in PBS. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Liquid: BT-015-GMP/LQ
| Formulation | Supplied as a 0.2 μm filtered solution in PBS. |
| Shipping | The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: IL-15
The dominant mechanism of IL-15 action is known as transpresentation in which IL-15 and IL-15 R alpha are coordinately expressed on the surface of one cell and interact with complexes of IL-2 R beta/ gammac on adjacent cells (7). This enables cells to respond to IL-15 even if they do not express IL-15 R alpha (6). In human and mouse, soluble IL-15-binding forms of IL-15 R alpha can be generated by proteolytic shedding and bind up nearly all the IL-15 in circulation (8-10). Soluble IL-15 R alpha functions as an inhibitor that limits IL-15 action (4, 9). Ligation of membrane-associated IL-15/IL-15 R alpha complexes also induces reverse signaling that promotes activation of the IL-15/IL-15 R alpha expressing cells (11). IL-15 induces or enhances the differentiation, maintenance, or activation of multiple T cell subsets including NK, NKT, Th17, Treg, and CD8+ memory cells (12 - 16). An important component of these functions is the ability of IL‑15 to induce dendritic cell differentiation and inflammatory activation (11, 14). IL-15 exhibits anti-tumor activity independent of its actions on NK cells or CD8+ T cells (17). It also inhibits the deposition of lipid in adipocytes, and its circulating levels are decreased in obesity (18).
Immunotherapy treatment with recombinant IL-15 has the
advantage of not stimulating Treg cells like IL-2 does but has the drawback of
associated toxicity at higher doses. This has led to increased investigation on
mitigating IL-15 toxicity and combination immunotherapy approaches using immune
checkpoint inhibitors (19, 20). Preclinical and
early clinical studies have shown the potential of also using IL-15 in
combination with cancer vaccines to improve their anti-tumor response (20).
IL-15 can also be used for the preconditioning of CAR T cells or for
engineering cells to express IL-15 in vivo. Adoptive cell transfer of NK cells
engineered to express CD19 and IL-15 were well tolerated in patients with
CD19-positive cancers (20).
IL-15 can be used in combination with other cytokines like
IL-21 to increase the efficiency of NK cell expansion and maturation in stem
cell culture protocols (21). The combination of IL-15 with IL-7 also promotes
expansion of early-differentiated CD8+ T cells in culture with the added benefit
of decreasing Treg cell generation, unlike IL-2, for adoptive cell transfer in
cancer immunotherapy (22). GMP IL-7 and GMP IL-15 are commonly used in
combination for ex vivo expansion of T cells for cellular therapies.
References
- De Sabatino, A. et al. (2011) Cytokine Growth Factor Rev. 22:19.
- Grabstein, K. et al. (1994) Science 264:965.
- Tagaya, Y. et al. (1997) Proc. Natl. Acad. Sci. USA 94:14444.
- Giri, J.G. et al. (1995) EMBO J. 14:3654.
- Giri, J. et al. (1994) EMBO J. 13:2822.
- Dubois, S. et al. (2002) Immunity 17:537.
- Castillo, E.F. and K.S. Schluns (2012) Cytokine 59:479.
- Budagian, V. et al. (2004) J. Biol. Chem. 279:40368.
- Mortier, E. et al. (2004) J. Immunol. 173:1681.
- Bergamaschi, C. et al. (2012) Blood 120:e1.
- Budagian, V. et al. (2004) J. Biol. Chem. 279:42192.
- Mortier, E. et al. (2003) J. Exp. Med. 205:1213.
- Gordy, L.E. et al. (2011) J. Immunol. 187:6335.
- Harris, K.M. (2011) J. Leukoc. Biol. 90:727.
- Xia, J. et al. (2010) Clin. Immunol. 134:130.
- Schluns, K.S. et al. (2002) J. Immunol. 168:4827.
- Davies, E. et al. (2010) J. Leukoc. Biol. 88:529.
- Barra, N.G. et al. (2010) Obesity 18:1601.
- Xue, D. et al. (2021) Antib Ther. 4:123.
- Wolfarth, A.A. et al. (2022) Immune Netw. 22:e5.
- Oberoi, P. et al. (2020) Cells. 9:811.
- Chamucero-Millares, J.A. et al. (2021) Cellular Immunol. 360:104257.
Long Name
Interleukin 15
Alternate Names
IL15
Entrez Gene IDs
Gene Symbol
IL15
UniProt
Additional IL-15 Products
Product Documents for Recombinant Human IL-15 GMP Protein, CF
Manufacturing Specifications
R&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.R&D Systems' quality focus includes:
- Manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented processes and QA control of documentation and process changes
- Personnel training programs
- Raw material testing and vendor qualification/monitoring
- Fully validated equipment, processes and test methods
- Equipment calibration schedules using a computerized calibration program
- Facility maintenance, safety programs and pest control
- Material review process for variances
- Monitoring of stability over product shelf-life
R&D Systems strives to provide our customers with the analytical characteristics of each product so that customers may determine whether our products are appropriate for their research. The Certificate of Analysis provided contains the following lot specific information:
- N-terminal amino acid analysis, SDS-PAGE analysis, and endotoxin level (as determined by LAL assay) performed on each bulk QC lot, not on individual bottlings of each QC lot
- Post-bottling lot-specific bioassay results (compliance with an established range) and results of microbial testing according to USP <71>
- Host Cell Protein testing performed by ELISA
- Mycoplasma testing by ribosomal RNA hybridization assay
Additional testing and documentation requested by the customer can be arranged at an additional cost.
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo cell therapy applications. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Animal-Free Manufacturing Conditions
Our dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers in a dedicated cold storage room.
Quality Assurance
- Low Endotoxin Level.
- No impairment of biological activity.
- High quality product obtained under stringent conditions.
Product Specific Notices for Recombinant Human IL-15 GMP Protein, CF
Full terms and conditions of sale can be found online in the Protein Sciences Segment T&Cs at: Terms & Conditions.
For preclinical, or clinical ex vivo use
Loading...
Loading...
Loading...