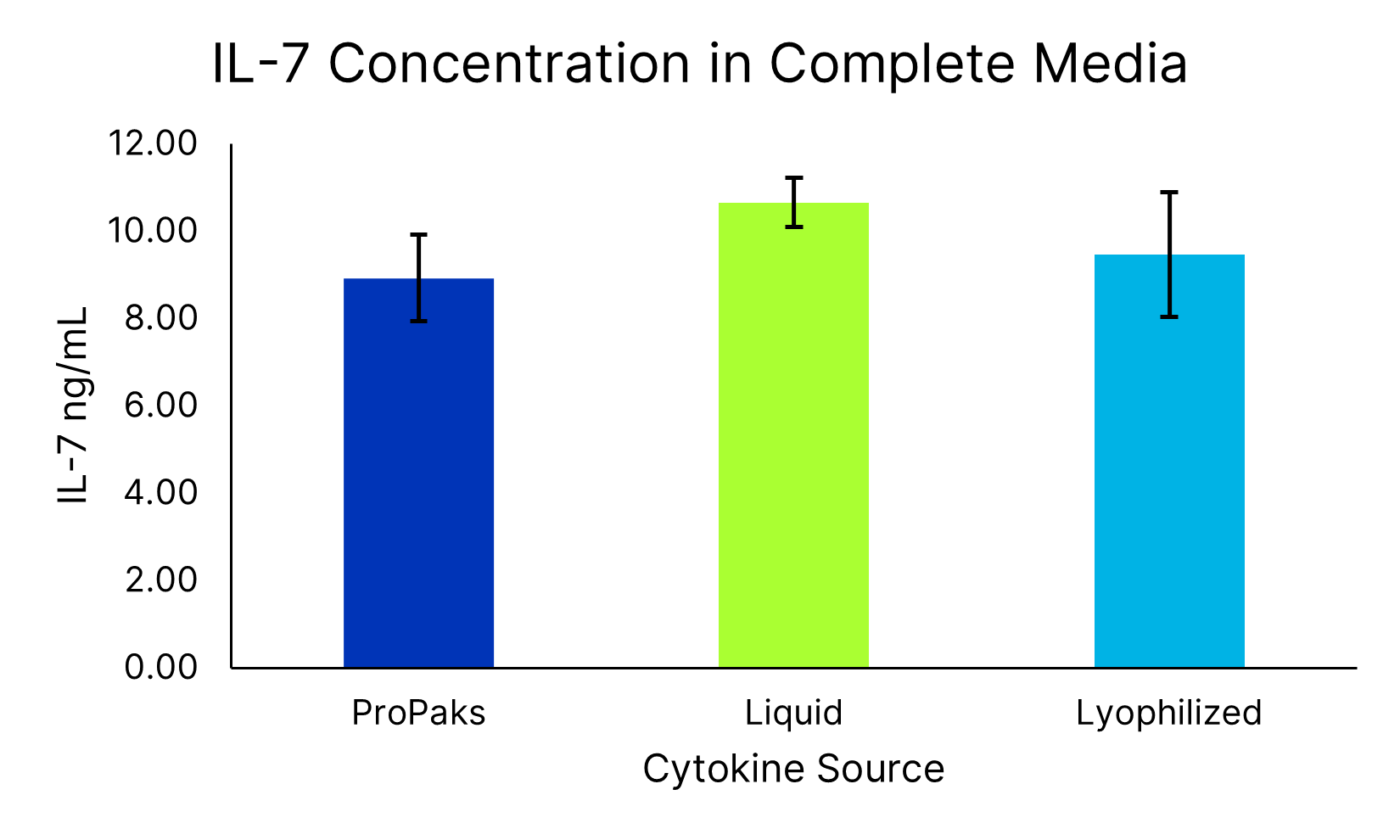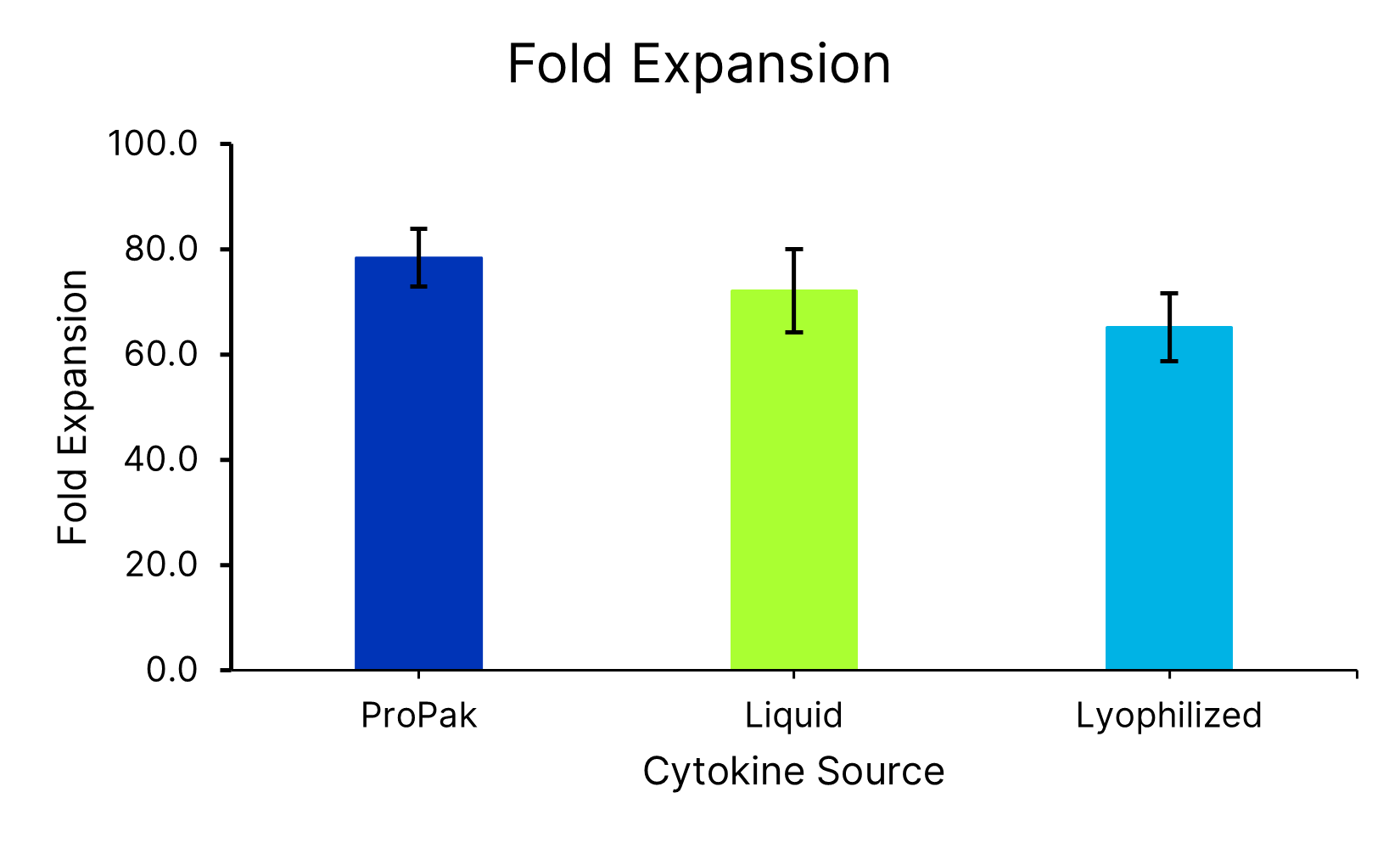ProPak™ Recombinant Human IL-7 GMP Protein GMP
R&D Systems, part of Bio-Techne | Catalog # PPK-007-GMP

Key Product Details
- Closed System Process In order to de-risk your therapy as much as possible, ProPak GMP cytokines are designed to integrate seamlessly into a closed-system process. The risk of contamination is significantly lowered as a result. Learn more about closed process reagents.
- Weldable Tubing Each ProPak bag is outfitted with weldable tubing, so you can connect it directly to your media, then add both to your bioreactor of choice. Tubing is compatible with any bagged media for use in a 1 L bioreactor, such as the G-Rex.
- Ready-to-Use Format Supplied in a 3mL frozen liquid format. Extensive quality testing guarantees that the liquid formulation is just as potent and stable as lyophilized equivalents, allowing you to safely take away the need for reconstitution steps.
- Reduced Manual Touchpoints By removing both reconstituting and aliquoting steps, there is less hands-on technician time. This drastically reduces risks of error while streamlining your journey to the clinic.
Product Specifications
Source
E. coli-derived human IL-7 protein
Asp26-His177, with an N-terminal Met
Produced using non-animal reagents in an animal-free laboratory.Manufactured and tested under cGMP guidelines.
Asp26-His177, with an N-terminal Met
Produced using non-animal reagents in an animal-free laboratory.Manufactured and tested under cGMP guidelines.
Purity
>97%, by SDS-PAGE with quantitative densitometry by Coomassie® Blue Staining. The molecular weight by mass spectrometry is 17507 Da ± 50 Da.
Endotoxin Level
<5.0 EU/mL by the LAL method.
N-terminal Sequence Analysis
Met-Asp26-(Cys)-Asp-Ile-Glu-Gly-Lys-Asp-Gly
Predicted Molecular Mass
17 kDa
SDS-PAGE
17 kDa, under reducing conditions.
Activity
Measured in a cell proliferation assay using PHA-activated human peripheral blood lymphocytes (PBL). Yokota, T. et al. (1986) Proc. Natl. Acad. Sci. USA 83:5894.
The ED50 for this effect is 0.100-0.500 ng/mL. The specific activity of Recombinant Human IL-7 is >1.00 x 108 units/mg, which is calibrated against the human IL-7 reference standard (NIBSC code: 90/530).
The ED50 for this effect is 0.100-0.500 ng/mL. The specific activity of Recombinant Human IL-7 is >1.00 x 108 units/mg, which is calibrated against the human IL-7 reference standard (NIBSC code: 90/530).
Host Cell Protein
<0.5 ng per μg of protein when tested by ELISA.
Mycoplasma
Negative when tested in a ribosomal RNA hybridization assay.
Host Cell DNA
<0.0015 ng per µg of protein when tested by PCR.
Scientific Data Images for ProPak™ Recombinant Human IL-7 GMP Protein
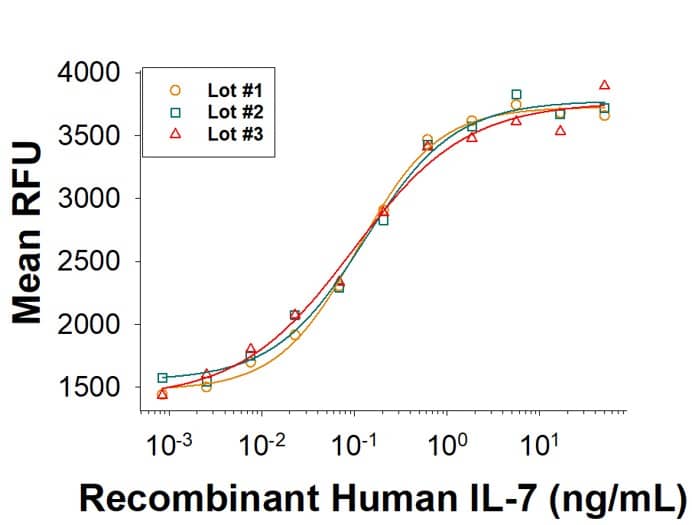
Lot to Lot Consistency
ProPak GMP-grade Recombinant Human IL-7 (PPK-007-GMP) stimulates proliferation of PHA-activated human peripheral blood lymphocytes. The ED50 for this effect is 0.100-0.500 ng/mL. Three independent lots were tested for activity and plotted on the same graph to show lot-to-lot consistency of ProPak GMP IL-7.ProPak cytokines are successfully recovered into 1L media bags.
ProPak IL-7 (PPK-007-GMP) and ProPak IL-15 (PPK-015-GMP) were added to Xeno-free Human T cell media in a bag (CCM038-GMP-1B). In parallel, liquid IL-7 (BT-007-GMP-025/LQ) and IL-15 (BT-015-GMP-025/LQ) or lyophilized IL-7 (BT-007-GMP) and IL-15 (BT-015-GMP) were added to Xeno-free Human T cell media in a bottle (CCM038-GMP-1L). After cytokine addition, the concentration of IL-7 (SPCKB-CS-003676) and IL-15 (SPCKB-CS-003688) was determined by the Ella automated immunoassay system. Data is the average of 4 independent experiments with error bars ±SD.ProPak cytokines support T cell growth
ProPak IL-7 (PPK-007-GMP) and ProPak IL-15 (PPK-015-GMP) were compared liquid IL-7 (BT-007-GMP-025/LQ) and IL-15 (BT-015-GMP-025/LQ) or lyophilized IL-7 (BT-007-GMP) and IL-15 (BT-015-GMP). Each were added to Xeno-free Human T cell media in a bag (CCM038-GMP-1B) with 5% huAB Serum. T cells from 3 donors were activated and expanded in G-Rex bioreactors for 9 days and assessed for fold expansion. Data is the average of 4 independent experiments with 3 donors per experiment, error bars ±SD.Formulation, Preparation and Storage
PPK-007-GMP
| Formulation | Supplied as a 0.2 μm filtered solution in PBS, recombinant HSA, and Trehalose. |
| Shipping | The product is shipped on dry ice. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles. A minimum of 6 months when stored between -14 °C and -40 °C. Can be stored up to 2 weeks at 2-8 °C. Refer to lot specific COA for the Use by Date. |
Background: IL-7
IL-7 is produced by a wide variety of cells in primary and secondary lymphoid tissues, including stromal epithelial cells of the thymus, bone marrow, and intestines (1, 2, 5). Circulating IL-7 is limiting in healthy animals, but increases during lymphopenia (1, 6). IL-7 signals through a complex of the IL-7 Receptor alpha subunit (IL-7 R alpha, also known as CD127) with the common gamma chain ( gammac) (1). The gammac is also a subunit of the receptors for IL-2, -4, -9, -15, and -21 (1).
IL-7 R alpha is expressed on double negative (CD4-CD8-) and single positive (CD4+ or CD8+) naïve and memory T cells, but undergoes IL-7-mediated down‑regulation and shedding during antigen-driven T cell proliferation, and is absent on regulatory T cells (1, 2, 6-11). IL-7 contributes to the maintenance of all naïve and memory T cells, mainly by promoting expression of the anti-apoptotic protein Bcl-2 (9-11). It is required for optimal T cell-dendritic cell interaction (6). IL-7 is expressed early in B cell development prior to the appearance of surface IgM (1, 5, 9). In mouse, IL-7 activation of IL-7 R alpha is critical for both T cell and B cell lineage development, while in humans, it is required for T cell but not for B cell development (4, 9, 12, 13). However, IL-7 functions in both mouse and human pro-B cells to suppress premature Ig light chain recombination during proliferative growth (14, 15).
Like other common gamma-chain cytokines like IL-2 and IL-15,
IL-7 and its receptor, IL-7R, has been used in a variety of immunotherapy
applications, often in fluid tumors and in some instances of solid tumor models
(16). Sometimes use of recombinant IL-7 is preferential as current studies and
early clinical trials of cancer have found less severe toxicity or side effects
upon treatment with IL-7 in comparison to IL-15 or IL-2 (16).
In CAR-T cell therapies, enhanced expression and secretion
of human IL-7 and CCL19 have enhanced the ability of T cells to expand and
migrate in vitro (17). Engineered CAR T cells expressing IL-7 or a
constitutively active IL-7R results in increased efficacy of CAR T anti-tumor
effects (16, 18). IL-7 is also frequently used in combination with IL-15 as a
supplement in cell culture of CAR T cells to support their expansion (19).
Additionally, IL-7/IL-15 in the presence of cord blood-derived T cells helps to
maintain their early differentiation state (20).
Monoclonal antibodies against IL-7R or small
molecule inhibitors against the IL-7R signaling pathway are commonly used in
circumstances of autoimmune diseases to delay disease progression (16). Also due to its ability to stimulate both
adaptive and innate immune cells, treatment with IL-7 has shown improved
survival in patients with sepsis who are at risk of deadly secondary infections
(21), providing evidence for IL-7 applications beyond cancer immunotherapy.
References
- Sasson, S.C. et al. (2006) Curr. Drug Targets 7:1571.
- Barata, J.T. et al. (2006) Exp. Hematol. 34:1133.
- Goodwin, R.G. et al. (1990) Proc. Natl. Acad. Sci. USA 86:302.
- Namen, A.E. et al. (1988) Nature 333:571.
- Shalapour, S. et al. (2012) PLoS ONE 7: e31939.
- Saini, M. et al. (2009) Blood 113:5793.
- Park, J.H. et al. (2004) Immunity 21:289.
- Vranjkovic, A. et al. (2007) Int. Immunol. 19:1329.
- Sudo, T. et al. (1993) Proc. Natl. Acad. Sci. 90:9125.
- Seddon, B. et al. (2003) Nat. Immunol. 4:680.
- Schluns, K.S. et al. (2000) Nat. Immunol. 5:426.
- Peschon, J.J. et al. (1994) J. Exp. Med. 180:1955.
- Pribyl, J.A. and T.W. LeBien (1996) Proc. Natl. Acad. Sci. 93:10348.
- Johnson, K. et al. (2012) J. Immunol. 188:6084.
- Nodland, S.E. et al. (2011) Blood 118:2116.
- Wang, C. et al. (2022) Int. J. Mol. Sci. 23:10370.
- Pang, N. et al. (2021) J Hematol Oncol. 14:118.
- Li, L. et al. (2022) Sci Rep. 12:12506.
- Xu, Y. et al. (2014) Blood. 123:3750.
- Marton,C. et al. (2022) Cancer Gene Ther. 29:961.
- Winer, H. et al. (2022) Cytokine. 160:156049.
Long Name
Interleukin 7
Alternate Names
IL7, Lymphopoietin-1, PBGF
Gene Symbol
IL7
UniProt
Additional IL-7 Products
Product Documents for ProPak™ Recombinant Human IL-7 GMP Protein
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Designed, manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented and controlled manufacturing process
- Control of documentation and process changes by QA
- Personnel training programs
- Raw material inspection and vendor qualification/monitoring program
- Validated equipment, processes and test methods
- Equipment calibration and maintenance schedules using a Regulatory Asset Manager
- Facility/Utilities maintenance, contamination controls, safety and pest control programs
- Material review process for variances
- Robust product stability program following relevant ICH guidelines
- N-terminal amino acid analysis
- SDS-PAGE purity analysis
- Molecular weight analysis via mass spectrometry
- Endotoxin assessment per USP <85> and Ph. Eur. 2.6.14 guidelines
- Bioassay analysis
- Microbial testing per USP <71> and Ph. Eur. 2.6.1 guidelines
- Host cell protein assessment
- Host cell DNA assessment
- Mycoplasma assessment
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis and St. Paul, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Animal-Free Manufacturing Conditions
Our dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers.
Product Specific Notices for ProPak™ Recombinant Human IL-7 GMP Protein
Full terms and conditions of sale can be found online in the Protein Sciences Segment T&Cs at: Terms & Conditions.
For preclinical, or clinical ex vivo use
Loading...
Loading...