By Victoria Osinski, PhD
What is the NLRP3 Inflammasome?
With its critical role in innate immunity, the nucleotide-binding oligomerization domain-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome is a sensor complex within cells that forms in response to endogenous danger signals, microbial motifs, and other environmental cues. The complex is made up of a sensor (NLRP3), an adaptor called the adaptor protein apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC), and an effector (caspase-1). Upon stimulation, NLRP3 oligomerizes, allowing recruitment of ASC. Multiple ASC filaments assemble into a structure known as a speck. From there, procaspase-1 can interact with ASC, self-cleave into active caspase-1 which induces activation and release of cytokines IL-1 and IL-18 through Gasdermin D pores.1 This NLRP3 complex has been shown to be involved in multiple diseases including Alzheimer’s disease, atherosclerosis, cryopyrin- associated periodic syndrome (CAPS), and diabetes.2,3
In addition to the canonical signaling pathway, the NLRP3 inflammasome can be activated through a non-canonical pathway involving caspases 4/-5 in humans and caspase-11 in mice, as well as an alternative inflammasome pathway that is independent of ASC speck formation and involves caspase-8 signaling.2
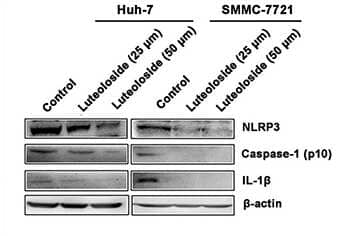
Western blot analysis of NLRP3 and other inflammasome-related components in human hepatoma Huh-7 and hepatocellular carcinoma SMMC-7721 cell lines. Control cells and cells were exposed luteoloside, a flavonoid with anti-inflammatory properties, were probed with Anti-Rabbit NLRP3/NALP3 Polyclonal Antibody (Catalog #NBP2-12446). Results showed reduced NLRP3 expression in treated cells. Data image submitted by a verified customer review.
NLRP3 Inflammasome Activation in Macrophages
Macrophages are an innate immune cell that influences the progression of many diseases and are found in tissues throughout the body. A number of stimuli have been shown to induce NLRP3 inflammasome formation in macrophages (and other cell types) including the commonly known Gram-negative bacterial outer membrane component, lipopolysaccharide (LPS), plus any of the following:
- nigericin (an antibiotic derived from Streptomyces hygroscopicus)
- ATP
- aluminum salts
- monosodium urate crystals
Multiple published studies have reported that components of specific pathogens activate NLRP3 through a variety of mechanisms. Lipoproteins from Mycoplasma salivarium and Mycoplasma pneumoniae were shown to induce IL-1 and IL-18 production via TLR2-NLRP3 in bone marrow-derived macrophages (BMDMs).4 Additionally, components from Staphylococcus aureus extracellular vesicles activated the NLRP3 inflammasome in both TLR2-dependent and -independent mechanisms depending on which components of the vesicles interacted with BMDMs.5 Interestingly, another study demonstrated that a non-pathogenic compound octanal, which binds the olfactory receptor 2, induced inflammatory responses in BMDMs in an OLFR2- and NLRP3-dependent manner. Treatment of atherosclerotic mice lacking ApoE with octanal exacerbated the development of plaques in major arteries.6
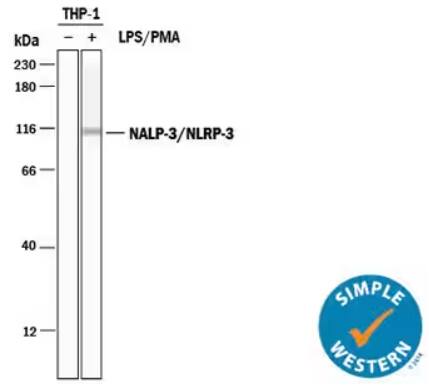
Simple Western lane view analysis depicting lysates from THP‑1 human acute monocytic leukemia cell line either untreated (-) or treated (+) with phorbol 12-myristate 13-acetate (PMA) and lipopolysaccharide (LPS). NLRP3/NALP3 was detected using Sheep Anti-Human NLRP3/NALP3 Polyclonal Antibody (Catalog # AF6789) followed by HRP-conjugated Anti-Sheep IgG Secondary Antibody (Catalog # HAF016) resulting in a specific band at ~111 kDa. The experiment was run on a 12-230 kDa separation system under reducing.
Besides the various pathogenic motifs and proteins, there are numerous other factors affecting NLRP3 activation in macrophages. One recent study demonstrated that NLRP3 inflammasome formation preferentially occurs when macrophages are cultured on compliant (or more elastic) surfaces in pro-inflammatory conditions.7 Furthermore, NLRP11, which is expressed in humans but not mice, is an essential part of the NLRP3 inflammasome in human macrophages.8 NLRP3 activation also depends on specific phosphorylation steps. A study recently demonstrated that phosphorylation of Ser725 by (Msn)/NIK-related kinase 1 (MINK1)/MAP4K6 was required for inflammatory responses in macrophages and in vivo.9
Macrophage-specific NLRP3 Inflammasomes Drive Inflammation in Multiple Disease Settings
To date, NLRP3 inflammasome activation has been implicated in multiple disease settings. In the context of pancreatic cancer, there is an observed enrichment of NLRP3+ macrophages in murine tumors. Global knockout of NLRP3 reduced tumor associated macrophage numbers and reprogrammed remaining cells toward a pro-inflammatory, tumor-suppressive phenotype and attenuated tumor growth.10 In a diabetic ischemic stroke setting (in which injury is driven by M1 macrophages)11, a handful of studies utilized NLRP3 inflammasome inhibitors CY-09 and MCC950 and found that these treatments restore heart function12 and brain function13, respectively. However, NLRP3 inflammasome in non-macrophage cell types such as cardiac fibroblasts can also regulate ischemic injury outcomes in the heart.14 Thus, further research is needed to pinpoint the extent to which macrophages influence outcomes both in pancreatic cancer and ischemic stroke.
Interestingly, murine models have been designed to express the mutant NLRP3A350V which was identified as a driver of CAPS. NLRP3A350V causes formation of hyperactive inflammasomes and resultant excess IL-1β and IL-18 expression. Using this model, researchers have investigated the cell type specific effects of this mutation in vivo. Expression of mutant NLRP3A350V in a general myeloid population (LysM-cre)15, macrophages (Fcgr1-cre)16, and neutrophils (Mrp8-cre)17 leads to multi-organ autoinflammation. These novel findings provide exciting, cell-specific data to link the roles of NLRP3 activation and macrophages in the progression of CAPS. Continued investigation using macrophage-specific drug delivery and genetic interventions will likely uncover additional pathogenic roles of NLRP3 inflammasome-driven activity in macrophages and other myeloid cells in various diseases.
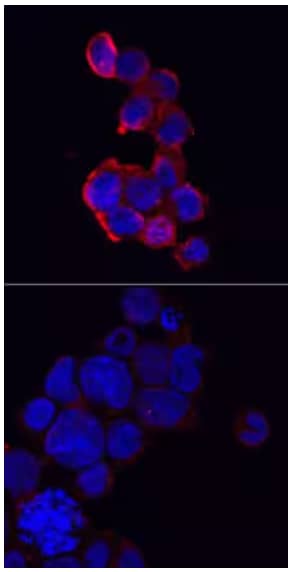
Immunocytochemistry analysis of NLRP3/NALP3 immersion fixed THP‑1 human acute monocytic leukemia cells in unstimulated (lower) and PMA- and LPS-stimulated cells (upper). Expression was detected using Sheep Anti-Human NLRP3/NALP3 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF6789) followed by staining with NL557-conjugated Anti-Sheep IgG Secondary Antibody (red; Catalog # NL010) and counterstained with DAPI (blue). Specific NLRP3 staining was localized to cytoplasm in the stimulated cells.

Victoria (Tori) Osinski, PhD
University of Minnesota Medical School
-
Shi, J. et al. (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death Nature 526:660-665.
-
Kelley, N. et al. (2019) The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation Int J Mol Sci 2019:3328.
-
Swanson, K.V. et al. (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics Nat Rev Immunol 19:477-489.
-
Saeki, A. et al. (2018) Activation of NLRP3 inflammasome in macrophages by mycoplasmal lipoproteins and lipopeptides Mol Oral Microbiol 33:300-311.
-
Wang, X. et al. (2020) Orchestration of human macrophage NLRP3 inflammasome activation by Staphylococcus aureus extracellular vesicles Proc Natl Acad Sci U S A 117:3174-3184.
-
Orecchioni, M. et al. (2022) Olfactory receptor 2 in vascular macrophages drives atherosclerosis by NLRP3-dependent IL-1 production Science 375:214-221.
-
Escolano, J.C. et al. (2021) Compliant Substrates Enhance Macrophage Cytokine Release and NLRP3 Inflammasome Formation During Their Pro-Inflammatory Response Front Cell Dev Biol 9:639815.
-
Gangopadhyay, A. et al. (2022) NLRP3 licenses NLRP11 for inflammasome activation in human macrophages Nat Immuno 23:892-903.
-
Zhu, K. et al. (2021) Priming of NLRP3 inflammasome activation by Msn kinase MINK1 in macrophages Cell Mol Immunol 18:2372-2382.
-
Daley, D. et al. (2017) NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma J Exp Med 214:1711-1724.
-
Liu, W. et al. (2015) Activation in M1 but not M2 Macrophages Contributes to Cardiac Remodeling after Myocardial Infarction in Rats: a Critical Role of the Calcium Sensing Receptor/NRLP3 Inflammasome Cell Physiol Biochem 35:2483-2500.
-
Lin, H.B. et al. (2020) Macrophage-NLRP3 Inflammasome Activation Exacerbates Cardiac Dysfunction after Ischemic Stroke in a Mouse Model of Diabetes Neurosci Bull 36:1035-1045.
-
Hong, P. et al. (2018) Inhibition of NLRP3 Inflammasome Ameliorates Cerebral Ischemia-Reperfusion Injury in Diabetic Mice Neural Plas
-
Sandanger, Ø. et al. (2013) The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury Cardiovasc Res 99:164-174.
-
Brydges, S.D. et al. (2009) Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity Immunity 30:875-887.
-
Frising, U.C. et al. (2022) Nlrp3 inflammasome activation in macrophages suffices for inducing autoinflammation in mice EMBO Rep
-
Stackowicz, J. et al. (2021) Neutrophil-specific gain-of-function mutations in Nlrp3 promote development of cryopyrin-associated periodic syndrome J Exp Med