Recombinant Human SCF GMP Protein, CF GMP
R&D Systems, part of Bio-Techne | Catalog # 255B-GMP

Key Product Details
Product Specifications
Source
Glu26-Ala189, with an N-terminal Met.
Produced using non-animal reagents in an animal-free laboratory.
Manufactured and tested under cGMP guidelines.
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
SDS-PAGE
Activity
The ED50 for this effect is 1‑5 ng/mL.The specific activity of recombinant human SCF is >1.0 x 106 units/mg, which is calibrated against the human SCF reference standard (NIBSC code: 91/682).
Host Cell Protein
Mycoplasma
Host Cell DNA
Scientific Data Images for Recombinant Human SCF GMP Protein, CF
Recombinant Human SCF GMP Protein Bioactivity
GMP-grade Recombinant Human SCF (Catalog # 255B-GMP) stimulates cell proliferation of the TF‑1 human erythroleukemic cell line. The ED50 for this effect is 1-5 ng/mL. Four independent lots were tested for activity and plotted on the same graph to show lot-to-lot consistency of GMP SCF.Equivalent Bioactivity of GMP and Animal-Free grades of Recombinant Human SCF
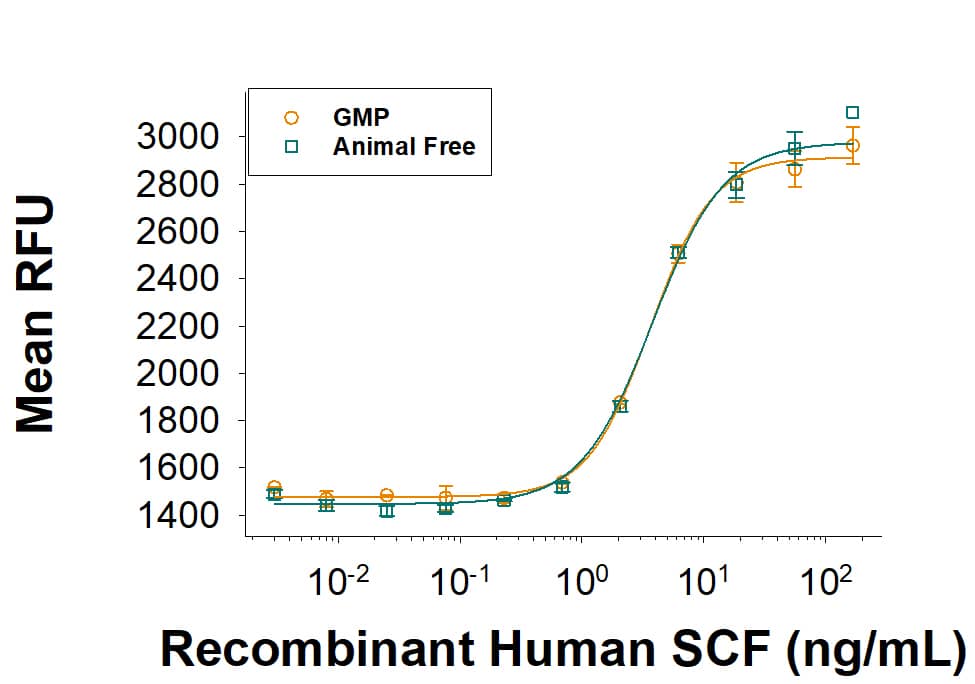
Equivalent bioactivity of GMP (Catalog # 255B-GMP) and Animal-Free (AFL255) grades of Recombinant Human SCF as measured in cell proliferation assay (orange and green respectively).Recombinant Human SCF GMP Protein SDS-PAGE
2 μg/lane of GMP-grade Recombinant Human SCF (Catalog # 255B-GMP) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing R band at 18 kDa and NR band at 16 kDa, respectively.Formulation, Preparation and Storage
255B-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution | Reconstitute at 100 μg/mL in PBS. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: SCF/c-kit Ligand
Stem cell factor (SCF), also known as c-kit ligand (KL), mast cell growth factor (MGF), and steel factor (SLF), is a widely expressed 28‑40 kDa type I transmembrane glycoprotein (1). It promotes the survival, differentiation, and mobilization of multiple cell types including myeloid, erythroid, megakaryocytic, lymphoid, germ cell, and melanocyte progenitors (1‑7). SCF is a primary growth and activation factor for mast cells and eosinophils (8). Mature human SCF consists of a 189 amino acid (aa) extracellular domain (ECD), a 23 aa transmembrane segment, and a 36 aa cytoplasmic tail (9). The ECD shows both N‑linked and O-linked glycosylation (10). Proteolytic cleavage at two alternate sites in the extracellular juxtamembrane region releases a 25 kDa soluble molecule which is comparable to the only form produced by Steel-dickie mutant mice (11, 12). An alternately spliced isoform of human SCF lacks 28 aa that encompasses the primary proteolytic recognition site (13). Within the ECD of the long isoform (corresponding to this recombinant protein), human SCF shares 79%‑87% aa sequence identity with canine, feline, mouse, and rat SCF. Rat SCF is active on mouse and human cells, but human SCF is only weakly active on mouse cells (9). Noncovalent dimers of transmembrane or soluble SCF interact with the receptor tyrosine kinase SCF R/c‑kit to trigger receptor dimerization and signaling (14). SCF assists in the recovery of cardiac function following myocardial infarction by increasing the number of cardiomyocytes and vascular channels (15).
References
- Ashman, L.K. (1999) Int. J. Biochem. Cell Biol. 31:1037.
- Sette, C. et al. (2000) Int. J. Dev. Biol. 44:599.
- Yoshida, H. et al. (2001) J. Invest. Dermatol. Symp. Proc. 6:1.
- Erlandsson, A. et al. (2004) Exp. Cell Res. 301:201.
- Kapur, R. et al. (2002) Blood 100:1287.
- Wang, C.-H. et al. (2007) Arterioscler. Thromb. Vasc. Biol. 27:540.
- Bashamboo, A. et al. (2006) J. Cell Sci. 119:3039.
- Reber, L. et al. (2006) Eur. J. Pharmacol. 533:327.
- Martin, F.H. et al. (1990) Cell 63:203.
- Arakawa, T. et al. (1991) J. Biol. Chem. 266:18942.
- Majumdar, M.K. et al. (1994) J. Biol. Chem. 269:1237.
- Brannan, C.I. et al. (1991) Proc. Natl. Acad. Sci. 88:4671.
- Anderson, D.M. et al. (1991) Cell Growth Differ. 2:373.
- Lemmon, M.A. et al. (1997) J. Biol. Chem. 272:6311.
- Kanellakis, P. et al. (2006) Cardiovasc. Res. 70:117.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional SCF/c-kit Ligand Products
Product Documents for Recombinant Human SCF GMP Protein, CF
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented processes and QA control of documentation and process changes
- Personnel training programs
- Raw material testing and vendor qualification/monitoring
- Fully validated equipment, processes and test methods
- Equipment calibration schedules using a computerized calibration program
- Facility maintenance, safety programs and pest control
- Material review process for variances
- Monitoring of stability over product shelf-life
- N-terminal amino acid analysis, SDS-PAGE analysis, mass spectrometry, and endotoxin level (as determined by LAL assay) performed on each bulk QC lot, not on individual bottlings of each QC lot
- Post-bottling lot-specific bioassay results (compliance with an established range) and results of microbial testing according to USP
- Host Cell Protein testing performed by ELISA
- Mycoplasma testing by ribosomal RNA hybridization assay
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Animal-Free Manufacturing Conditions
Our dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers in a dedicated cold storage room.
- Low Endotoxin Level.
- No impairment of biological activity.
- High quality product obtained under stringent conditions.
Product Specific Notices for Recombinant Human SCF GMP Protein, CF
Full terms and conditions of sale can be found online in the Protein Sciences Segment T&Cs at: Terms & Conditions.
For preclinical, or clinical ex vivo use