Human Chemerin Antibody
R&D Systems, part of Bio-Techne | Catalog # MAB2324

Key Product Details
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Glu21-Ser157
Accession # Q99969
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human Chemerin Antibody
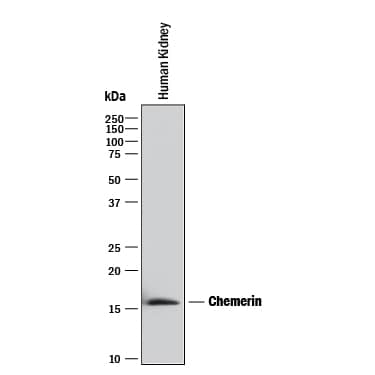
Detection of Human Chemerin by Western Blot.
Western blot shows lysates of human kidney tissue. PVDF membrane was probed with 2 µg/mL of Mouse Anti-Human Chemerin Monoclonal Antibody (Catalog # MAB2324) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (HAF018). A specific band was detected for Chemerin at approximately 16 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.Detection of Chemerin by Western Blot
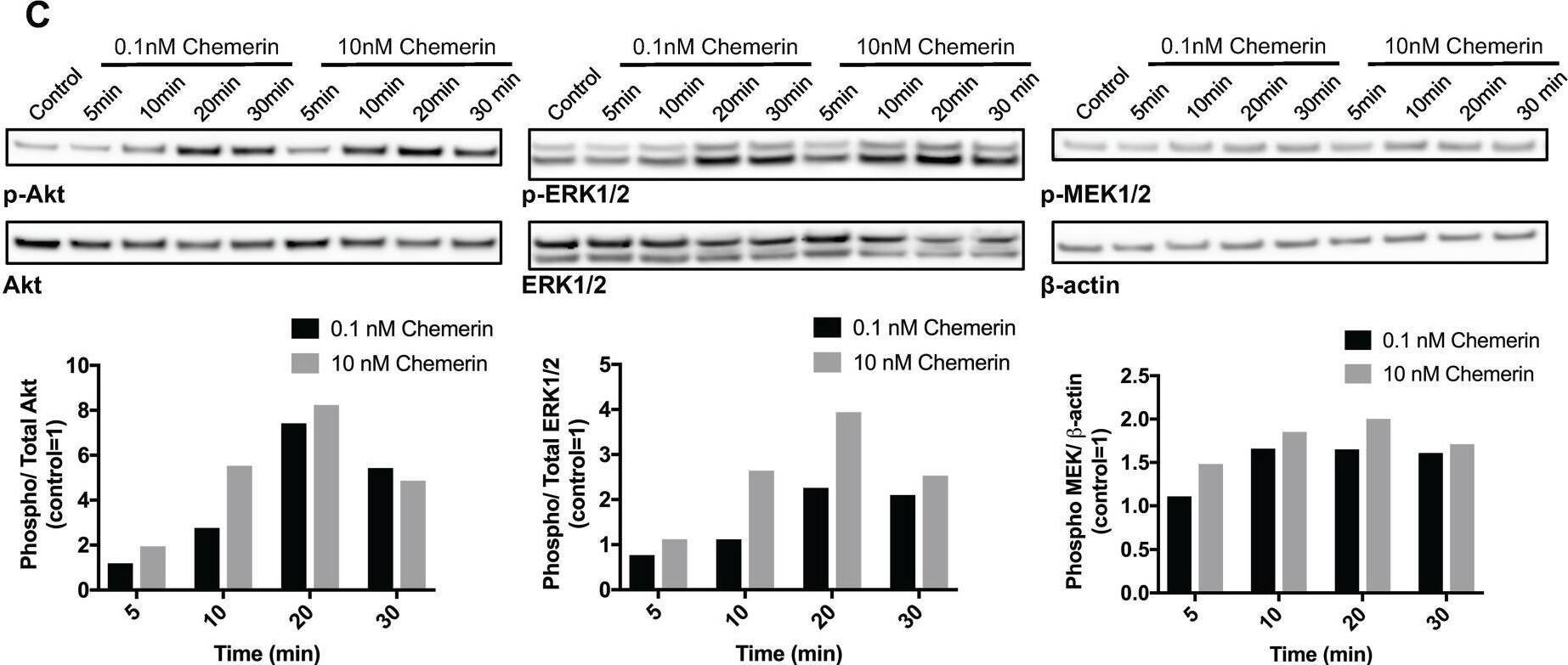
Chemerin induces intracellular calcium mobilization and stimulates MAPK and Akt signaling in neuroblastoma cells Intracellular calcium mobilization was measured in SK-N-SH cells with confocal laser scanning microscopy following the stimulation with 10nM chemerin without (A) and with (B) the prior addition of the calcium chelator EDTA. The arrow indicates the time point when chemerin was added. (C) Chemerin stimulates the phosphorylation of Akt, ERK1/2 and MEK1/2 in SK-N-AS cells in a dose-dependent manner. The cells were serum-starved for 24h prior to stimulation and samples were taken 5, 10, 20 and 30min after stimulation. Densitometric analysis of the protein bands was performed and the ratios between p-ERK1/2 and total ERK1/2, p-Akt and total Akt as well as p-MEK1/2 and beta-actin were calculated. The values are displayed relative to the control=1. The experiments were performed three times with similar results. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29221117), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of Chemerin by Western Blot
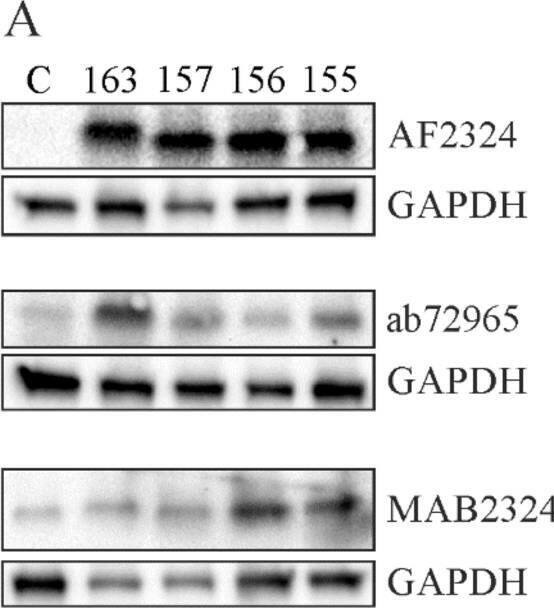
Analysis of chemerin variants by immunoblot. (A) Human chemerin isoforms 163, 157, 156 and 155 were overexpressed in HepG2 cells and protein was detected by three different antibodies (C, control-transfected cells). (B) Quantification of chemerin protein (n = 2). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/33066325), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Human Chemerin Antibody
Western Blot
Sample: Human kidney tissue
Formulation, Preparation, and Storage
Purification
Reconstitution
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Chemerin
Human Chemerin, also known as Tazarotene-induced Gene 2, (TIG2) is a new, but distant member of the Cystatin superfamily (1-3). Members of this superfamily contain at least two intrachain disulfide bonds and an alpha-helical structure over a distance of about 100 amino acids (2, 3). Chemerin is synthesized as a 163 aa precursor that contains a hydrophobic 20 aa N-terminal sequence, an intervening 137 aa Cystatin-fold containing domain, and a six aa C-terminal prosegment (1, 4). Within the cystatin-fold domain there are three intrachain disulfide bonds that contribute to the fold, and three potential sites for phosphorylation and one for myristoylation (5). The precursor molecule undergoes proteolytic processing at both termini by unknown proteases. The N-terminal residue 20 aa hydrophobic segment is described as being either a signal sequence or a transmembrane (TM) segment for a type II TM protein (1, 6). In either case, it gives rise to a soluble proform that undergoes further processing at the C-terminus. In human, the C-terminal six residues are cleaved, giving rise to a monomeric, 16 kDa heparin-binding bioactive molecule (aa 21-157) (7). A shorter 134 aa form has been described (5). Bioactivity seems to be concentrated in the nine residues preceding the prosegment (aa 149‑157). Retention of the prosegment blocks activity (4). The 137 aa mature segment is known to bind to the G-protein coupled receptor termed ChemR23 (5, 7). Binding results in macrophage and immature dendritic cell chemotaxis (7). The distribution of this receptor is limited to immune APCs, and it is assumed that Chemerin is an inflammatory molecule. It is unclear which cells are actually producing Chemerin, but keratinocytes, endothelial cells and osteoclasts are potential candidates (1, 7). Mature human Chemerin shares 67% aa sequence identity with mouse Chemerin (7). There is apparently cross-species activity for the protein (8).
References
- Nagpal, S. et al., (1997) J. Invest. Dermatol. 109:91.
- Storici, P. et al., (1996) Eur. J. Biochem. 238:769.
- Zanetti, M., (2004) J. Leukoc. Biol. 75:39.
- Wittamer, V. et al., (2004) J. Biol. Chem. 279:9956.
- Meder, W. et al., (2003) FEBS Lett. 555:495.
- Yokoyama-Kobayashi, M. et al., (1999) Gene 228:161.
- Wittamer, V. et al., (2003) J. Exp. Med. 198:977.
- Busmann, A. et al., (2004) J. Chromatog. B 811:217.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional Chemerin Products
Product Documents for Human Chemerin Antibody
Product Specific Notices for Human Chemerin Antibody
For research use only