Ubiquitin: Small Molecules and Peptides
Ubiquitin is a 76 amino acid (aa) protein that is ubiquitously expressed in all eukaryotic organisms. Ubiquitin is highly conserved with 96% aa sequence identity shared between human and yeast Ubiquitin, and 100% aa sequence identity shared between human and mouse Ubiquitin. In mammals, four Ubiquitin genes encode for two Ubiquitin-ribosomal fusion proteins and two poly-Ubiquitin proteins. Cleavage of the Ubiquitin precursors by deubiquitinating enzymes gives rise to identical Ubiquitin monomers each with a predicted molecular weight of 8.6 kDa. Conjugation of Ubiquitin to target proteins involves the formation of an isopeptide bond between the C-terminal glycine residue of Ubiquitin and a lysine residue in the target protein. This process of conjugation, referred to as ubiquitination or ubiquitylation, is a multi-step process that requires three enzymes: a Ubiquitin-activating (E1) enzyme, a Ubiquitin-conjugating (E2) enzyme, and a Ubiquitin ligase (E3). Ubiquitination is classically recognized as a mechanism to target proteins for degradation and as a result, Ubiquitin was originally named ATP-dependent Proteolysis Factor 1 (APF-1). In addition to protein degradation, ubiquitination has been shown to mediate a variety of biological processes such as signal transduction, endocytosis, and post-endocytic sorting.
2 results for "Ubiquitin Small Molecules and Peptides" in Products
2 results for "Ubiquitin Small Molecules and Peptides" in Products
Ubiquitin: Small Molecules and Peptides
Ubiquitin is a 76 amino acid (aa) protein that is ubiquitously expressed in all eukaryotic organisms. Ubiquitin is highly conserved with 96% aa sequence identity shared between human and yeast Ubiquitin, and 100% aa sequence identity shared between human and mouse Ubiquitin. In mammals, four Ubiquitin genes encode for two Ubiquitin-ribosomal fusion proteins and two poly-Ubiquitin proteins. Cleavage of the Ubiquitin precursors by deubiquitinating enzymes gives rise to identical Ubiquitin monomers each with a predicted molecular weight of 8.6 kDa. Conjugation of Ubiquitin to target proteins involves the formation of an isopeptide bond between the C-terminal glycine residue of Ubiquitin and a lysine residue in the target protein. This process of conjugation, referred to as ubiquitination or ubiquitylation, is a multi-step process that requires three enzymes: a Ubiquitin-activating (E1) enzyme, a Ubiquitin-conjugating (E2) enzyme, and a Ubiquitin ligase (E3). Ubiquitination is classically recognized as a mechanism to target proteins for degradation and as a result, Ubiquitin was originally named ATP-dependent Proteolysis Factor 1 (APF-1). In addition to protein degradation, ubiquitination has been shown to mediate a variety of biological processes such as signal transduction, endocytosis, and post-endocytic sorting.
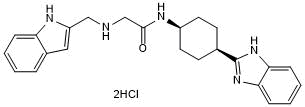
Selective GID4 antagonist
| Chemical Name: | N-((1s,4s)-4-(1H-Benzo[d]imidazol-2-yl)cyclohexyl)-2-(((1H-indol-2-yl)methyl)amino)acetamide dihydrochloride |
| Purity: | ≥98% (HPLC) |
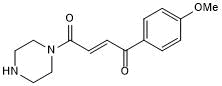
RNF126 chemical handle
| Chemical Name: | 1-(4-Methoxyphenyl)-4-(1-piperazinyl)-2-butene-1,4-dione hydrochloride |
| Purity: | ≥95% (HPLC) |