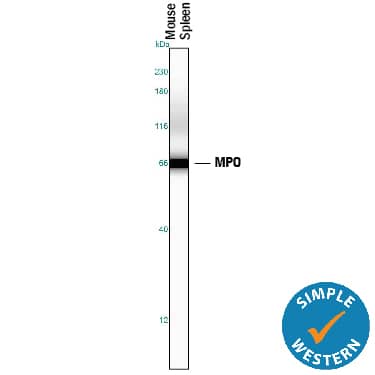
Detection of Mouse Myeloperoxidase/MPO by Simple WesternTM.
Simple Western lane view shows lysates of mouse spleen tissue, loaded at 0.2 mg/mL. A specific band was detected for Myeloperoxidase/MPO at approximately 67 kDa (as indicated) using 5 µg/mL of Goat Anti-Human/Mouse Myeloperoxidase/MPO Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3667) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (
HAF109). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
Detection of Human Myeloperoxidase/MPO by Immunohistochemistry
SAA levels positively correlate with tumour-associated neutrophil infiltration. (A) Representative images of MPO-positive neutrophils in a lung adenocarcinoma tumour section (original magnification ×200). (B) Neutrophils are significantly elevated in tumours compared to control tissue biopsies, which was confirmed by (C) paired analysis of tumour and adjacent control lung tissue. (D) Circulating SAA levels were elevated in lung adenocarcinoma patients as determined by ELISA. (E) SAA transcript levels were not increased in tumours as measured by RTqPCR and confirmed by (F) sub-analysis of the paired from the tumour and adjacent control tissue samples. (G) Tissue sections were stained for SAA, which identified positive staining within seromucinous glands and tumour-associated macrophages (original magnification ×200). (H) Spearman correlation was used to assess associations between SAA transcript levels and tumour infiltrating neutrophil density (r = 0.51, *** p < 0.001). **** p < 0.0001, ** p < 0.01, ## p < 0.01. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/34203378), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Myeloperoxidase/MPO by Immunocytochemistry/Immunofluorescence
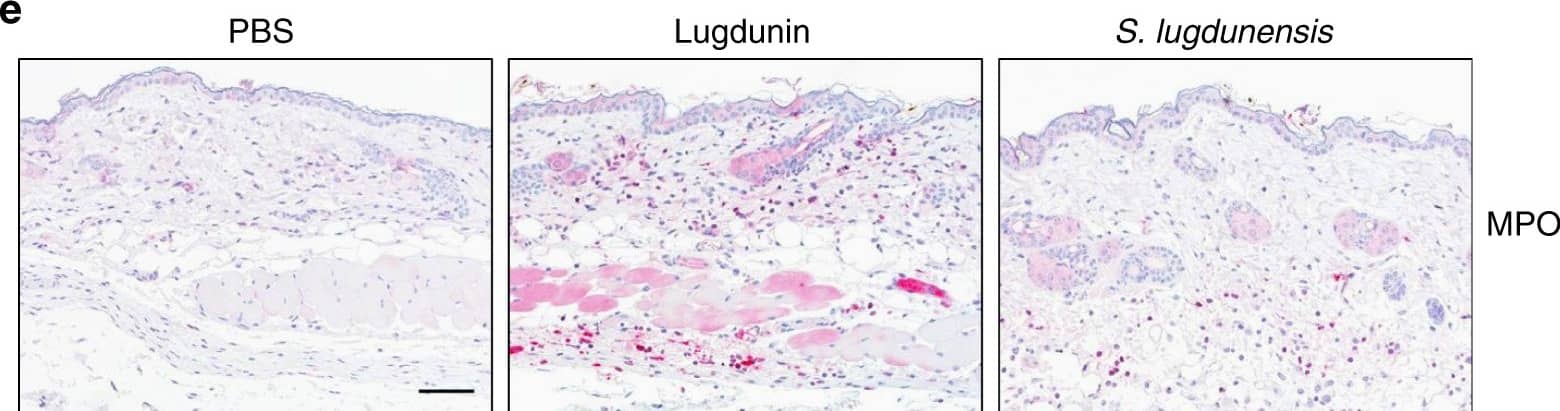
Epicutaneous lugdunin recruits phagocytic cells. a Schematic overview of the mouse experiments: 6–8-week-old female C5BL/6 wild-type (WT), MyD88-ko, or 5xTLR-ko mice were epicutaneously treated with 1.5 µg lugdunin or phosphate-buffered saline (PBS) as a control. After 24 h, mice were euthanized, immune cells were isolated from treated skin areas, and immune cell composition was analyzed by flow cytometry. b Shown is the mean percentage of CD45+ live cells in mouse skin of 10 C57BL/6 WT mice ± s.e.m. One mouse is represented as two dots analyzed by two different stainings. c Pie charts show the mean percentage of the different immune cell subsets in the skin of 10 WT mice after 24 h of PBS or lugdunin treatment. d Shown are representative flow cytometry data (left panel) and the mean percentage of neutrophils (Ly6C+Ly6G+) and monocytes (Ly6C+Ly6G−) pregated on CD11b+CD45+ live cells (see Supplementary Fig. 3a, f for the gating strategy) in mouse skin ± s.e.m. One dot represents one mouse. *P < 0.05. e Representative myeloperoxidase (MPO)-stained paraffin-embedded mouse skin sections. Scale bar, 100 µM. Source data are provided as a Source Data file Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31227691), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Myeloperoxidase/MPO by Western Blot
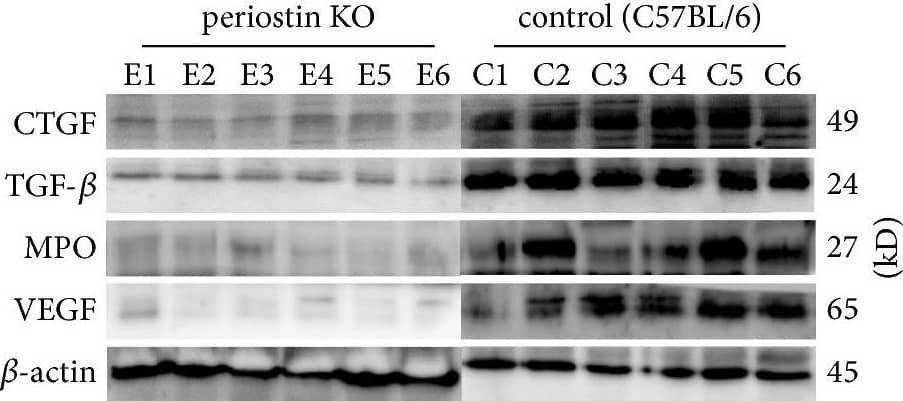
Levels of CTGF, TGF-beta, MPO, and VEGF in silicone implant-induced capsular tissues determined by western blotting (a). A low signal was obtained for CTGF (b) and TGF-beta (c) protein in PN-KO mice (n = 6), whereas a strong signal was detected in the C57BL/6 mice (n = 6). Compared with the control group (n = 6), the levels of MPO (d) and VEGF (e) protein were downregulated in the PN-KO group (n = 6). Relative expression levels normalized to the housekeeping gene beta-actin. ∗∗p < 0.001. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29854742), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Mouse Myeloperoxidase/MPO by Western Blot
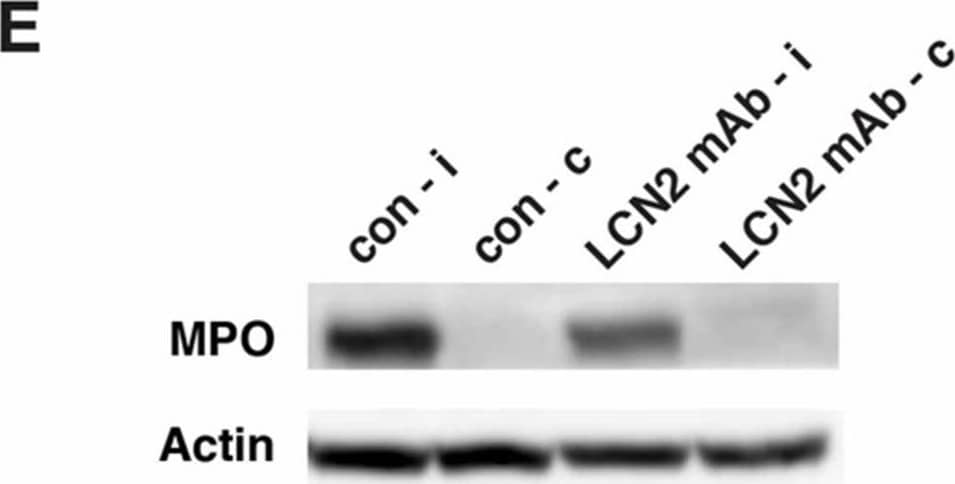
LCN2 mAb limited blood–brain barrier leakage and infiltration of neutrophils after tMCAo. Representative images (A) and quantification (B) of Evans blue extravasation in the ipsilateral hemispheres of mice treated with control IgG (con) and LCN2 mAb (n = 5 per group) after one hour of tMCAo and 23 h after reperfusion. The concentration of Evans blue dye in the ipsilateral hemispheres of mice treated with LCN2 mAb was significantly decreased (** p < 0.01) as compared with that in the ipsilateral hemispheres of mice treated with control IgG (two-tailed, unpaired t test); (C) The expression level of the tight junction protein claudin-5 was analyzed after treatments with control IgG and LCN2 mAb (n = 4 per group). The ipsilateral (i) and contralateral (c) hemispheres isolated at 23 h after tMCAo were analyzed by Western blotting using antibodies against claudin-5. Representative Western blot showing the expression of claudin-5 (~22 kDa) in brain homogenates. beta-actin served as a loading control; (D) The level of claudin-5 immunoreactivity normalized to beta-actin (claudin-5/actin) in the ipsilateral hemispheres in mice treated with LCN2 mAb was significantly higher than that in the ipsilateral hemispheres of mice that received the control IgG (* p < 0.05, one-tailed, unpaired t test); (E,F) Neutrophil infiltration was analyzed by measuring the levels of MPO in brain homogenates. The ipsilateral (i) and contralateral (c) hemispheres of mice treated with control IgG (con) and LCN2 mAb (n = 4 per group) isolated at 23 h after tMCAo were analyzed by Western blotting using antibodies against MPO; (E) Representative Western blots show the expression of MPO heavy chain (~55 kD) in brain homogenates; (F) The level of MPO immunoreactivity normalized to beta-actin (MPO/actin) was significantly reduced in the ipsilateral hemispheres of mice treated with LCN2 mAb (* p < 0.05, one-tailed, unpaired t test) as compared with that in the ipsilateral hemisphere of mice that received control IgG. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32872405), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Myeloperoxidase/MPO in Mouse Spleen.
Formalin-fixed paraffin-embedded tissue sections of mouse spleen were probed for MPO mRNA (ACD RNAScope Probe, catalog #603091; Fast Red chromogen, ACD catalog # 322750). Adjacent tissue section was processed for immunohistochemistry using goat anti-mouse MPO polyclonal antibody (R&D Systems catalog #
AF3667) at 1ug/mL with overnight incubation at 4 degrees Celsius followed by incubation with anti-goat IgG VisUCyte HRP Polymer Antibody (Catalog #
VC004) and DAB chromogen (yellow-brown). Tissue was counterstained with hematoxylin (blue). Specific staining was localized to cell surface.
Detection of Human Human/Mouse Myeloperoxidase/MPO Antibody by Immunohistochemistry
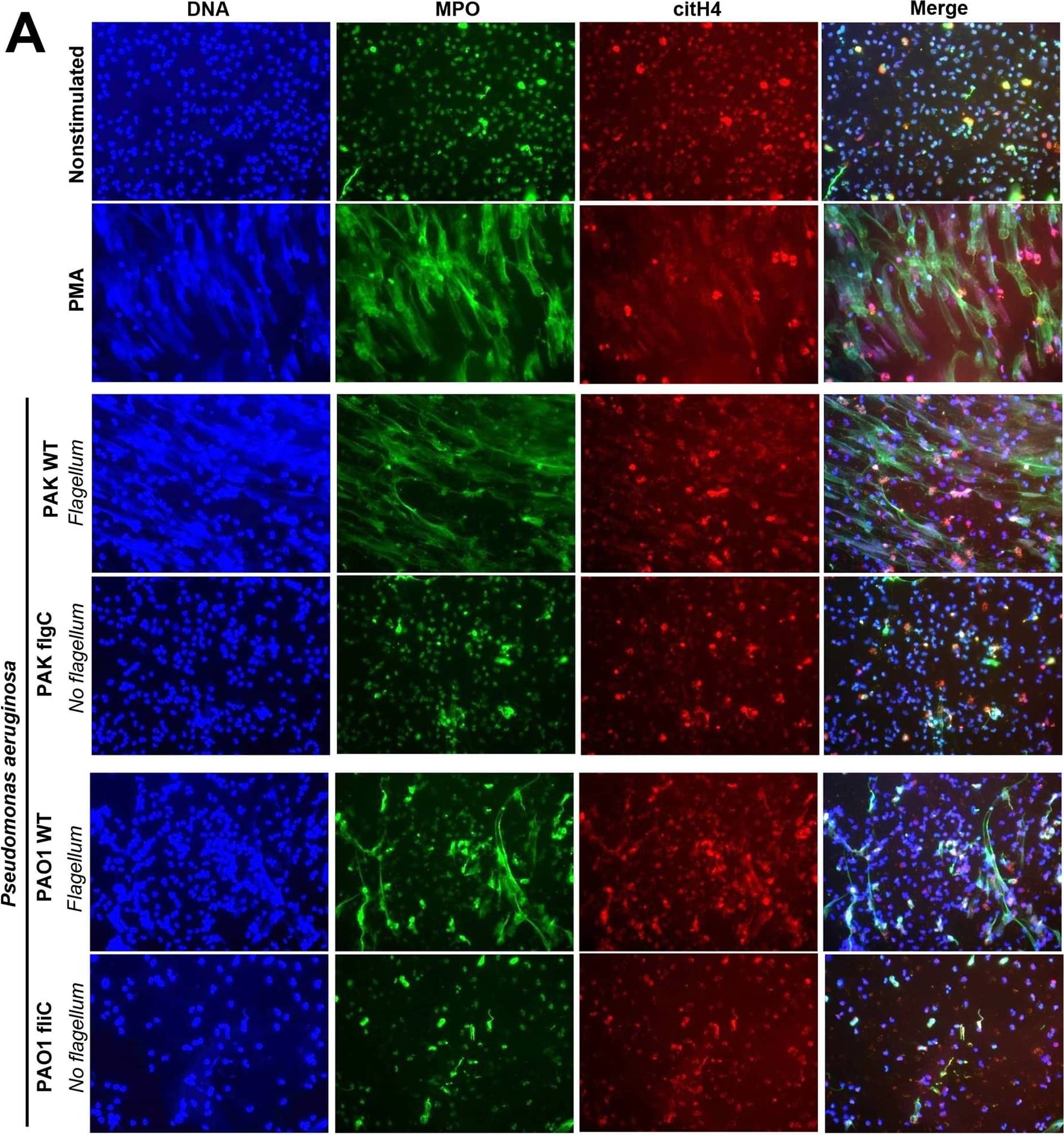
Immunofluorescence staining reveals that flagellum-deficiency abolishes P. aeruginosa-induced NET formation.Human PMNs were exposed to wild-type (WT) or flagellum-deficient strains of P. aeruginosa PAK flgC and PAO1 fliC, and NET release was documented. (A) Immunofluorescence staining of myeloperoxidase (MPO, green), citrullinated histone H4 (citH4, red), extracellular DNA (DAPI, blue) and their merged images are shown. Representative results, n = 3. (B) Quantitation of immunofluorescence images shown in panel (A). Based on nuclear morphology and co-staining with MPO and citH4, NET-forming PMNs were identified and their numbers quantitated compared to the total cell population (expressed as percentage of total). Mean+/-S.E.M., n = 3. One-way ANOVA, Tukey’s post hoc test. *, p<0.05; **, p<0.01. Nonstim., nonstimulated; WT, wild-type. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27855208), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human Myeloperoxidase/MPO by Immunohistochemistry
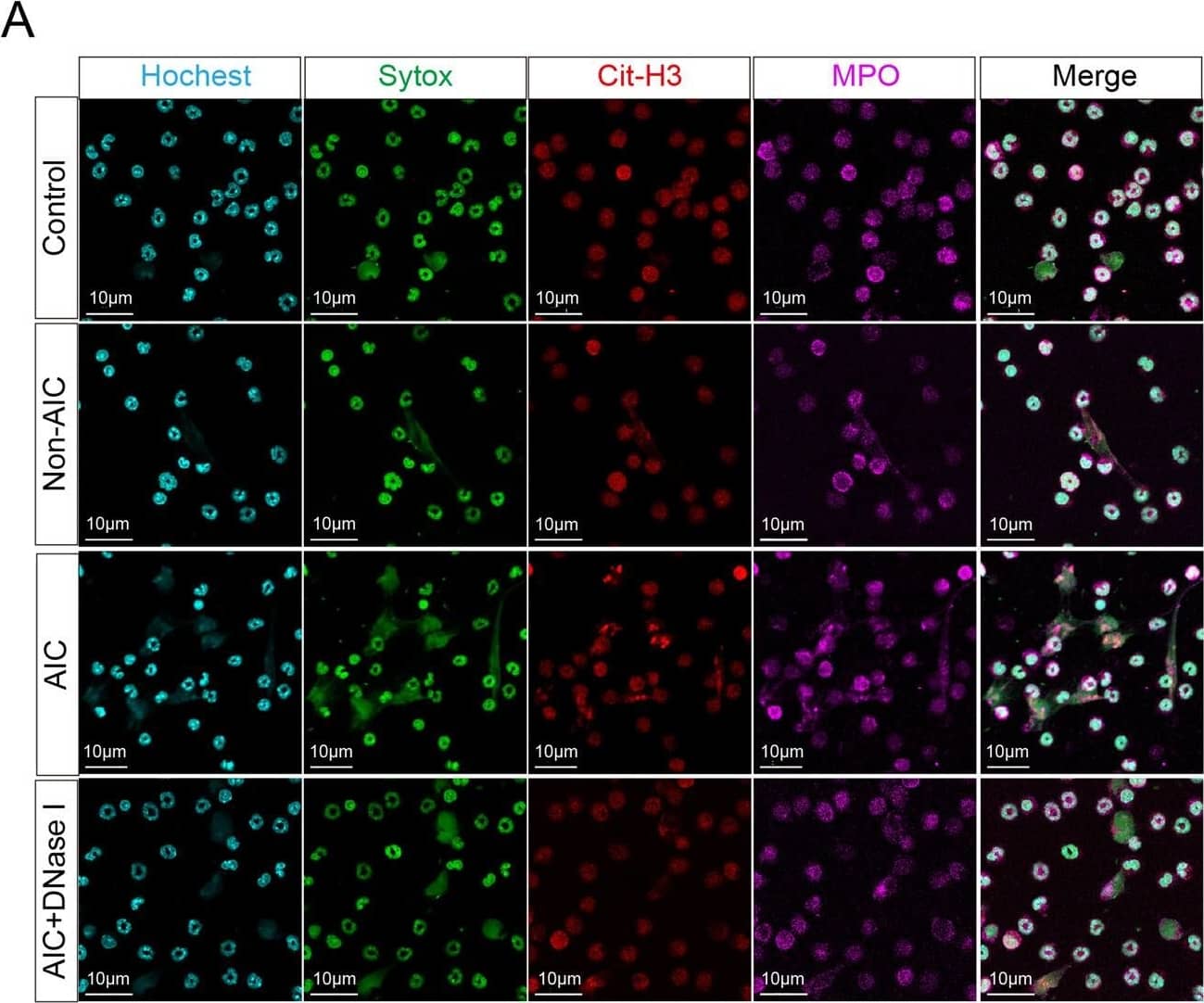
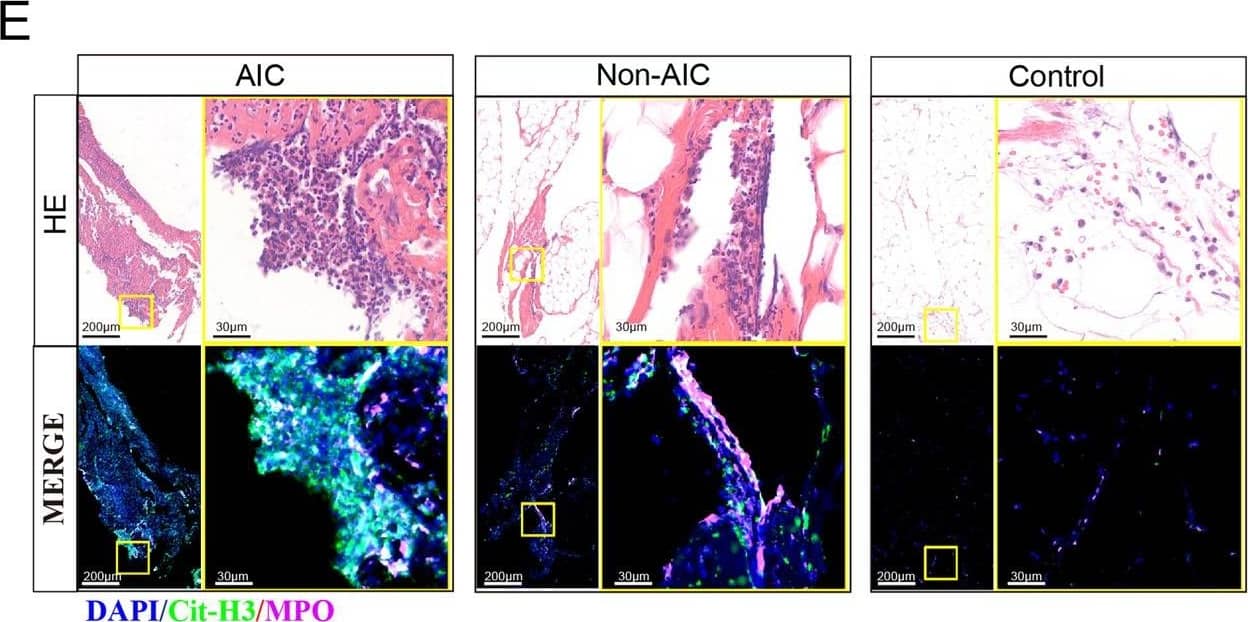
Postoperative AIC stimulates NETs release both in peripheral blood and abdominal infectious site.A Left panel: representative immunofluorescence co-staining of DNA (Hochest and Sytox), Cit-H3 (citrullinated histone-3) and MPO (myeloperoxidase) to assess NETs formation in the neutrophils isolated from peripheral blood of control, Non-AIC, AIC and AIC + DNase I groups; Right panel: plasma and serum levels of MPO–DNA in GC patients with control, Non-AIC and AIC; B Preoperative and postoperative serum MPO–DNA levels between Non-AIC and AIC groups; C Representative SEM (scan electron microscopy) of neutrophils isolated from preoperative and postoperative ascites fluid between Non-AIC and AIC groups. Green arrows point to extracellular meshes of NETs and white arrows point to neutrophils; D Preoperative and postoperative ascites fluid MPO–DNA levels between Non-AIC and AIC groups; E Representative images of HE and immunofluorescence staining of DNA, Cit-H3, and MPO in omental tissues among AIC, Non-AIC, and control groups; F Quantification of NETs in omental tissues among AIC, Non-AIC, and control groups. Data represent the mean ± S.D. in A, F (n = 10 per group); one-way ANOVA with Tukey test was used in A, F; paired and unpaired Student’s t-tests were used in B, D (n = 10 per group). Source data are provided as a Source Data file. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35197446), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human Myeloperoxidase/MPO by Immunohistochemistry
Postoperative AIC stimulates NETs release both in peripheral blood and abdominal infectious site.A Left panel: representative immunofluorescence co-staining of DNA (Hochest and Sytox), Cit-H3 (citrullinated histone-3) and MPO (myeloperoxidase) to assess NETs formation in the neutrophils isolated from peripheral blood of control, Non-AIC, AIC and AIC + DNase I groups; Right panel: plasma and serum levels of MPO–DNA in GC patients with control, Non-AIC and AIC; B Preoperative and postoperative serum MPO–DNA levels between Non-AIC and AIC groups; C Representative SEM (scan electron microscopy) of neutrophils isolated from preoperative and postoperative ascites fluid between Non-AIC and AIC groups. Green arrows point to extracellular meshes of NETs and white arrows point to neutrophils; D Preoperative and postoperative ascites fluid MPO–DNA levels between Non-AIC and AIC groups; E Representative images of HE and immunofluorescence staining of DNA, Cit-H3, and MPO in omental tissues among AIC, Non-AIC, and control groups; F Quantification of NETs in omental tissues among AIC, Non-AIC, and control groups. Data represent the mean ± S.D. in A, F (n = 10 per group); one-way ANOVA with Tukey test was used in A, F; paired and unpaired Student’s t-tests were used in B, D (n = 10 per group). Source data are provided as a Source Data file. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35197446), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Rat Myeloperoxidase/MPO by Immunohistochemistry
CXCR4 expressed at neutrophils mediates NET formation in rats. (D) Representative images of immunolabeling with CitH3, MPO,&DAPI at 20 mm distal to the injury site 12 h after injury. Dashed lines indicate the border of the epineurium&the parenchyma. * are high-magnification images of boxed areas in the epineurium. Arrows indicate triple immunolabeled NETs. Scale bar: 10 μm. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35961782), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Rat Myeloperoxidase/MPO by Immunohistochemistry
Migration inhibitory factor (MIF) secreted from neutrophils promotes NET formation in rats. (D) Representative images of immunolabeling with CitH3, MPO,&DAPI at 20 mm distal to the injury site 12 h after injury. Dashed lines indicate the border of the epineurium&the parenchyma. Images marked with * are high-magnification images of boxed areas in the epineurium. Arrows indicate triple immunolabeled NETs. Treatment with iso-1 dramatically inhibited NET formation. Scale bar: 10 μm. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35961782), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Rat Myeloperoxidase/MPO by Immunohistochemistry
Inhibition of NET formation promotes the macrophage infiltration from the epineurium into the parenchyma in rats. (C) Representative images of immunolabeling with CitH3, MPO,&DAPI at 20 mm distal to the injury site. Dashed lines indicate the border of the epineurium&the parenchyma. Images marked with * are high-magnification views of the boxed areas at the epineurium. Arrows indicate neutrophils releasing NETs. Treatment with Cl-amidine or DNase I inhibited NET formation. Scale bars: 100&10 μm in low-&high-magnification images, respectively. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35961782), licensed under a CC-BY license. Not internally tested by R&D Systems.
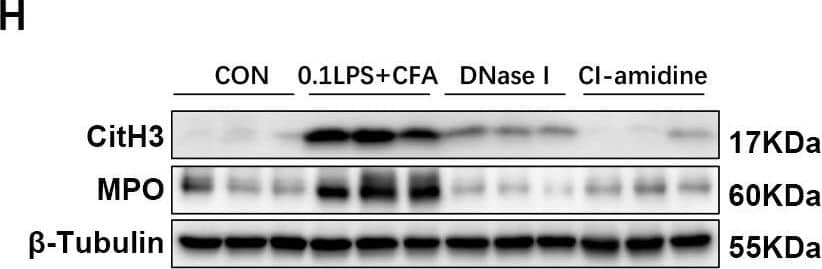
Detection of Myeloperoxidase/MPO by Western Blot
DNase I or CI-amidine administration reduced airway hyperresponsiveness and alleviated airway inflammation (A) Experimental assay schematic for in vivo experiments. (B) AHR was measured 24 h after the last challenge, enhanced pause (Penh) values were used as an indicator of lung function. (C) Hematoxylin and eosin (H&E) staining of lung tissue. Scale bar = 50 μm. (D) Paraffin acid-Schiff (PAS) staining of lung. Scale bar = 20 μm. (E) Quantification of inflammation infiltration score of the H&E staining. (F) Quantification of mucus-producing goblet cells of the PAS staining. (G) The total number of cells and the differential number of cells (eosinophils and neutrophils) were quantified 48 h after the last challenge in the BALF. (H, I) Western blot analysis to measure MPO and CitH3 protein expression level in lung tissue of four groups of mice. Expression is relative to beta-Tubulin. Cropped blots are shown, and supplementary Fig. S3 and S6 presents the full-length blots. Data were shown as mean ± SEM; n = 4–6. Significance between groups was calculated using one-way ANOVA with Tukey’s post hoc method. *p < 0.05, **p < 0.01 and ***p < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36271366), licensed under a CC-BY license. Not internally tested by R&D Systems.
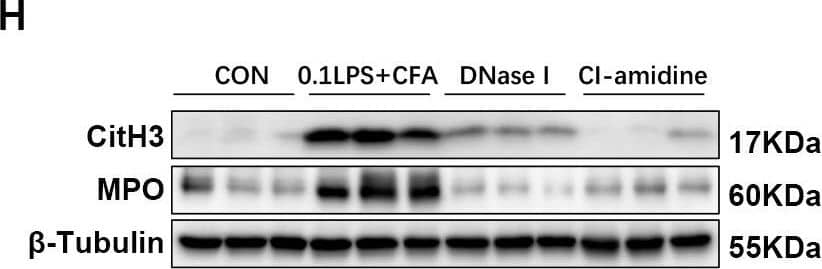
Detection of Myeloperoxidase/MPO by Western Blot
DNase I or CI-amidine administration reduced airway hyperresponsiveness and alleviated airway inflammation (A) Experimental assay schematic for in vivo experiments. (B) AHR was measured 24 h after the last challenge, enhanced pause (Penh) values were used as an indicator of lung function. (C) Hematoxylin and eosin (H&E) staining of lung tissue. Scale bar = 50 μm. (D) Paraffin acid-Schiff (PAS) staining of lung. Scale bar = 20 μm. (E) Quantification of inflammation infiltration score of the H&E staining. (F) Quantification of mucus-producing goblet cells of the PAS staining. (G) The total number of cells and the differential number of cells (eosinophils and neutrophils) were quantified 48 h after the last challenge in the BALF. (H, I) Western blot analysis to measure MPO and CitH3 protein expression level in lung tissue of four groups of mice. Expression is relative to beta-Tubulin. Cropped blots are shown, and supplementary Fig. S3 and S6 presents the full-length blots. Data were shown as mean ± SEM; n = 4–6. Significance between groups was calculated using one-way ANOVA with Tukey’s post hoc method. *p < 0.05, **p < 0.01 and ***p < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36271366), licensed under a CC-BY license. Not internally tested by R&D Systems.
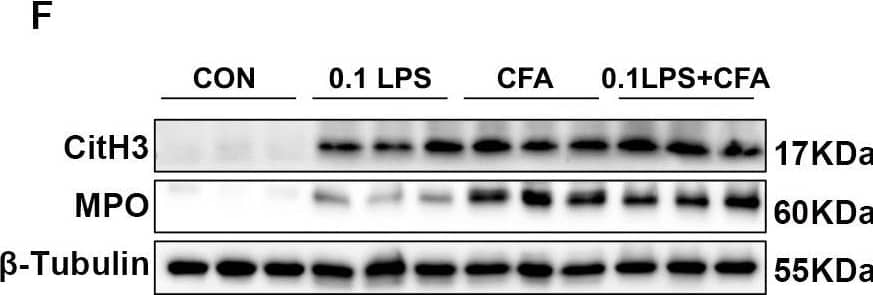
Detection of Myeloperoxidase/MPO by Western Blot
Neutrophilic mouse model induced by CFA combined with LPS showed enhanced NETs formation capacity. (A) At 48 h after the last challenge, the percentage of neutrophil populations in CD45(+) leukocytes in mouse peripheral blood and bone marrow were determined by flow cytometry. The representative images in each group are shown. (B) Statistical analysis of the percentage of CD11b(+)Ly6G(+) neutrophils in CD45(+) leukocyte gate of mouse peripheral blood and bone marrow by flow cytometry. (C) Neutrophils were purified from mouse bone marrow and stimulated with PMA (100 nM) or vehicle control for 4 h. Then, neutrophils were stained for myeloperoxidase (MPO, red), citrullinated histone 3 (CitH3, green), and DNA (DAPI, blue) and confocal by immunofluorescence microscope for analysis. Representative images of NETs immunofluorescence. Scale bar = 20 μm. (D) Percentage of NETs area normalized to MPO positive signal in mouse bone marrow neutrophils after PMA stimulation. (E) Representative z-axis images of the NETs immunofluorescence in 0.1LPS + CFA group. Scale bar = 10 μm. (F, G) Western blot analysis the protein expression level of MPO and CitH3 in lung tissue of four groups of mice. Expression is relative to beta-Tubulin. Cropped blots are shown, and supplementary Fig. S2 and S5 presents the full-length blots. Data were shown as mean ± SEM; n = 4 or 6. Significance between groups was calculated using one-way ANOVA with Tukey’s post hoc method. *p < 0.05, **p < 0.01 and ***p < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36271366), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Myeloperoxidase/MPO by Western Blot
Abundant NETs occur in neutrophilic asthma models. (A) Neutrophil expression in CD45(+) leucocytes in mouse peripheral blood and bone marrow were determined by flow cytometry within 48 h after the last challenge. The representative images in each group are shown. (B) Quantification: the percentage of CD11b(+)Ly6G(+) neutrophils in the CD45(+) leucocytes gate of mouse peripheral blood and bone marrow by flow cytometry. (C) In vitro NET-formation assays with purified neutrophils of mouse bone marrow neutrophils stimulated with PMA (100 nM) or RPMI 1640 for 4 h. Then the neutrophils were stained PI and analyzed by immunofluorescence confocal microscopy. Representative immunofluorescence images of NETs, Scale bar = 20 μm. (D) Comparison of the percentage of NETosis cells in each group upon PMA stimulation and control (Ctrl) stimulation. (E) After stimulated with PMA, the neutrophils were stained for myeloperoxidase (MPO, red), citrullinated histone 3 (CitH3, green) and DAPI (nuclear staining, blue) and analyzed by immunofluorescence confocal microscopy. Representative immunofluorescence images of NETs, Scale bar = 20 μm. (F) Percentage of NETs area normalized to MPO positive signal in mouse bone marrow neutrophils after PMA stimulation. (G, H) Western blot was used to detect the levels of MPO and CitH3 protein in lung tissue of five groups of mice. Expression is relative to beta-Tubulin. Cropped blots are shown, and supplementary Fig. S1 and S4 presents the full-length blots. Data were shown as mean ± SEM; n = 4. Significance between groups was calculated using one-way ANOVA with Tukey’s post hoc method. *p < 0.05, **p < 0.01 and ***p < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36271366), licensed under a CC-BY license. Not internally tested by R&D Systems.
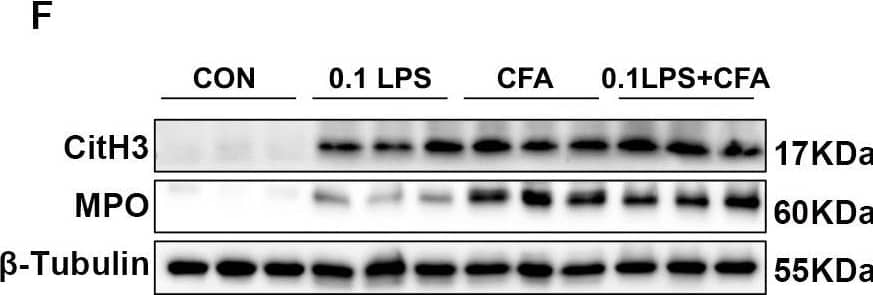
Detection of Myeloperoxidase/MPO by Western Blot
Neutrophilic mouse model induced by CFA combined with LPS showed enhanced NETs formation capacity. (A) At 48 h after the last challenge, the percentage of neutrophil populations in CD45(+) leukocytes in mouse peripheral blood and bone marrow were determined by flow cytometry. The representative images in each group are shown. (B) Statistical analysis of the percentage of CD11b(+)Ly6G(+) neutrophils in CD45(+) leukocyte gate of mouse peripheral blood and bone marrow by flow cytometry. (C) Neutrophils were purified from mouse bone marrow and stimulated with PMA (100 nM) or vehicle control for 4 h. Then, neutrophils were stained for myeloperoxidase (MPO, red), citrullinated histone 3 (CitH3, green), and DNA (DAPI, blue) and confocal by immunofluorescence microscope for analysis. Representative images of NETs immunofluorescence. Scale bar = 20 μm. (D) Percentage of NETs area normalized to MPO positive signal in mouse bone marrow neutrophils after PMA stimulation. (E) Representative z-axis images of the NETs immunofluorescence in 0.1LPS + CFA group. Scale bar = 10 μm. (F, G) Western blot analysis the protein expression level of MPO and CitH3 in lung tissue of four groups of mice. Expression is relative to beta-Tubulin. Cropped blots are shown, and supplementary Fig. S2 and S5 presents the full-length blots. Data were shown as mean ± SEM; n = 4 or 6. Significance between groups was calculated using one-way ANOVA with Tukey’s post hoc method. *p < 0.05, **p < 0.01 and ***p < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36271366), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Myeloperoxidase/MPO by Western Blot
Abundant NETs occur in neutrophilic asthma models. (A) Neutrophil expression in CD45(+) leucocytes in mouse peripheral blood and bone marrow were determined by flow cytometry within 48 h after the last challenge. The representative images in each group are shown. (B) Quantification: the percentage of CD11b(+)Ly6G(+) neutrophils in the CD45(+) leucocytes gate of mouse peripheral blood and bone marrow by flow cytometry. (C) In vitro NET-formation assays with purified neutrophils of mouse bone marrow neutrophils stimulated with PMA (100 nM) or RPMI 1640 for 4 h. Then the neutrophils were stained PI and analyzed by immunofluorescence confocal microscopy. Representative immunofluorescence images of NETs, Scale bar = 20 μm. (D) Comparison of the percentage of NETosis cells in each group upon PMA stimulation and control (Ctrl) stimulation. (E) After stimulated with PMA, the neutrophils were stained for myeloperoxidase (MPO, red), citrullinated histone 3 (CitH3, green) and DAPI (nuclear staining, blue) and analyzed by immunofluorescence confocal microscopy. Representative immunofluorescence images of NETs, Scale bar = 20 μm. (F) Percentage of NETs area normalized to MPO positive signal in mouse bone marrow neutrophils after PMA stimulation. (G, H) Western blot was used to detect the levels of MPO and CitH3 protein in lung tissue of five groups of mice. Expression is relative to beta-Tubulin. Cropped blots are shown, and supplementary Fig. S1 and S4 presents the full-length blots. Data were shown as mean ± SEM; n = 4. Significance between groups was calculated using one-way ANOVA with Tukey’s post hoc method. *p < 0.05, **p < 0.01 and ***p < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36271366), licensed under a CC-BY license. Not internally tested by R&D Systems.