Biopharmaceutical development is known to be a slow and expensive process, requiring deep pockets, unquestionable expertise, and infrastructure that houses sophisticated equipment. The era of speed and convenience that we live in, however, has continued to provide us with faster, robust, and more powerful tools in nearly all aspects of life, and the lab is no exception. While drug development will always require multiple analytical methods for thorough characterization, there is a rapidly growing effort towards building platforms that can perform multiple functions in an accelerated manner. The benefits conferred by such analytical platforms are many: consolidation of workflows into a single instrument, fewer capital investments, less training on a host of instruments, and streamlined method development. This article discusses the advantages of the MauriceFlex™ System, which is designed for comprehensive, high-quality analysis of biotherapeutics through charge (icIEF), charge fractionation, and size (CE-SDS) interrogation methods.
Three Workflows, One Platform
- icIEF delivers high-resolution charge variant analysis, a regulatory expectation for biologics development.
- CE-SDS provides robust size characterization, replacing gel-based methods with automated, quantitative workflows.
- icIEF fractionation takes charge variant analysis a step further, allowing users to collect charge fractions for
- Platform-agnostic downstream MS characterization without the need for buffer exchange.
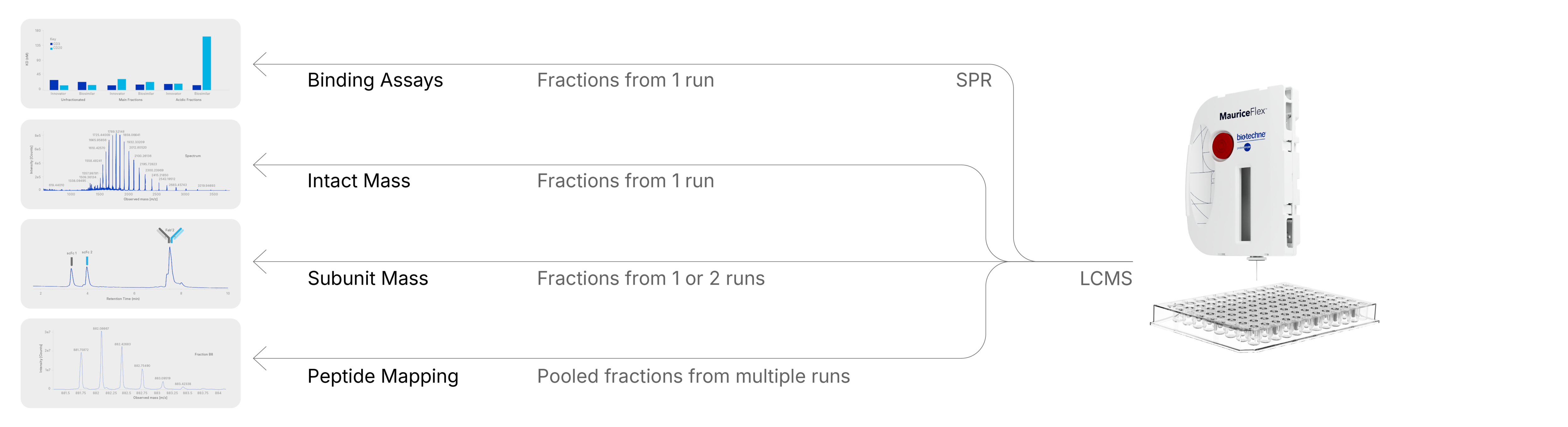
- Binding potency analysis using digital Surface Plasmon Resonance (SPR)

By enabling icIEF-based fractionation, the MauriceFlex system allows same-day separation and collection of charge isoforms, presenting significant time and labor savings from the otherwise traditional IEX workflow—that typically has longer method development times. In other words, MauriceFlex delivers IEX-level insights, with significantly less time, effort, and sample preparation needed for MS analysis.
Because downstream characterization requirements can vary based on the phase of drug development, instrument, and expertise availability, fractions collected on MauriceFlex are indeed flexible and can be analyzed on a variety of MS instruments, with little to no sample prep.
- LC-MS workflows for intact or subunit mass analysis
The MauriceFlex and BioAccord™ LC-MS system (Waters) were used together to analyze the differences between the innovator drug Belimumab and a research-grade biosimilar1. By directly loading the collected acidic, main, and basic fractions onto the LC-MS system for intact mass analysis, critical differences between the two molecules were observed, including the presence of C-terminal lysine, amino acid clipping, and deamidation. Another case study delves into subunit analysis of the acidic, main, and basic peaks of Mosunetuzumab, which is a bispecific antibody, and a research-grade biosimilar2. In this study, subunit analysis was performed by using a digestive enzyme called FabRICATOR® (Genovis) before loading on the BioAccord System. Most notably, LC-MS analysis clearly showed incorrect pairing of the light and heavy chains in the biosimilar, thus underscoring the need for such in-depth analysis of charge variant fractions.
- Microfluidic MS platforms like ZipChip for rapid, high-throughput characterization
Another collaborative study highlighted how MauriceFlex fractions can be paired with the ZipChip-MS system (908 Devices) for rapid charge variant characterization3. In this work, acidic, main, and basic fractions from an antibody-drug conjugate (ADC) parent mAb were collected using MauriceFlex and analyzed without post-run sample preparation. The ZipChip-MS workflow, which uses capillary electrophoresis coupled directly to mass spectrometry, enabled fast and sensitive intact mass analysis of individual charge variants. Results revealed major glycoforms and minor isoforms that aligned closely with reported mass data, confirming the ability of the icIEF fractionation + ZipChip-MS combination to deliver high-quality results in a fraction of the time required for traditional IEX workflows. This collaboration underscored the versatility of MauriceFlex fractions for use across different MS platforms, reinforcing its value as an MS-agnostic solution for biopharmaceutical characterization.
Beyond Molecular Structure: Insights into Biological Function
Importantly, charge heterogeneity doesn’t just affect molecular structure but can impact biological function too. In another collaborative study, the MauriceFlex system was used to fractionate acidic, main, and basic variants of the bispecific antibody Mosunetuzumab and a research-grade biosimilar, which were then analyzed for binding using the Alto™ Digital SPR System (Nicoya)4. Despite requiring only 2 µL of each fraction, the SPR assay provided detailed kinetic and affinity data for binding to CD3 and CD20 ligands. The results revealed that while most fractions displayed comparable binding across innovator and biosimilar, the acidic fraction of the biosimilar showed significantly weaker binding to CD20. These findings correlated with the aforementioned intact LC-MS study that had shown incorrect assembly in the same acidic fraction, as further evidence of how the combined icIEF fractionation and SPR workflow offers a powerful way to link structural heterogeneity with functional impact directly.
Continued Impact on the Field
A recent study by Wittmann et al. evaluated the suitability of the MauriceFlex platform for offline MS analysis5. Using Matuzumab as the analyte, charge analysis and fraction collection were completed in just 2.5 hours, with further characterization done on a QTOF MS system with an ESI source. The study offers a positive conclusion and states that “This cIEF fractionation with offline MS approach provides excellent selectivity and sensitivity for thoroughly investigating all charge variants of pharmaceutical monoclonal antibodies. The straightforward method development and reliable technical setup enable platform methods to effectively characterize these crucial substances during drug development”. Another study published in Electrophoresis by McElroy et al. demonstrated method optimization for fractionation of AAV charge variants with MauriceFlex, making significant inroads in AAV capsid protein characterization6.
Maximizing Value from a Single Instrument
In an environment where resources, time, and expertise are often stretched thin, MauriceFlex provides a clear advantage with simplified workflows and high-quality data offering deeper insights, all through a single platform. By integrating icIEF, CE-SDS, and icIEF fractionation, and enabling seamless connections to MS and SPR, MauriceFlex empowers scientists with a multi-attribute analysis tool that characterizes biotherapeutics with precision and efficiency, surpassing the capabilities of traditional workflows.
References
- Application Note: Charge Variant Characterization of Innovator & Biosimilar Drugs with MauriceFlex & BioAccord LC-MS System
- Application Note: Comparing Charge Variants: Innovator vs Biosimilar Using the MauriceFlex System & Mass Spectrometry
- Scientific Poster: Novel icIEF Fractionation Coupled with Different MS Systems for Rapid Charge Variant Characterization of Therapeutic Antibodies
- Application Note: A Novel icIEF Fractionation & SPR-Based Workflow for Correlating the Charge Structure to the Function of a Bispecific Antibody
- McElroy, W., Huang, S., He, X., Zhou, C., Heger, C. D., Powers, T. W., Anderson, M. M., Sloan, C., & Lerch, T. F. (2025). Enabling icIEF Peak Identification of AAV Capsid Proteins by Fractionation on MauriceFlex and Subsequent Analysis by LC-MS. Electrophoresis, 46(1-2), 22–33. https://doi.org/10.1002/elps.202400201
- Wittmann, A., Wilke, Y., Grammel, N., & Wätzig, H. (2025). Evaluation of a cIEF Fractionation Workflow for Offline MS Analysis of Charge Variants of the Monoclonal Antibody Matuzumab. Electrophoresis, 46(3-4), 240–249. https://doi.org/10.1002/elps.8108