Detection of Human IL-32 by Western Blot
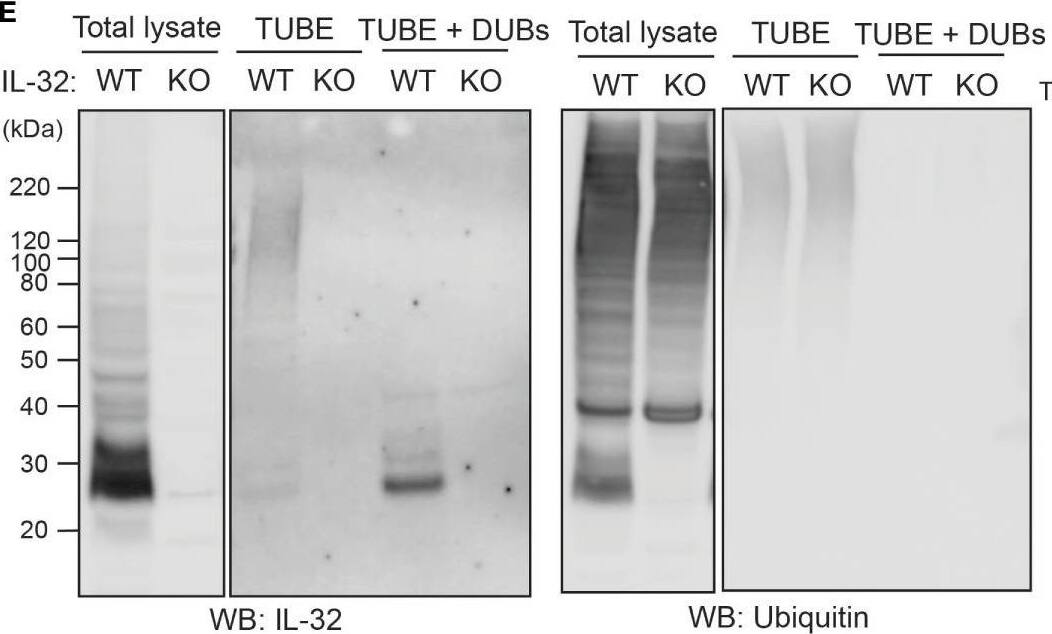
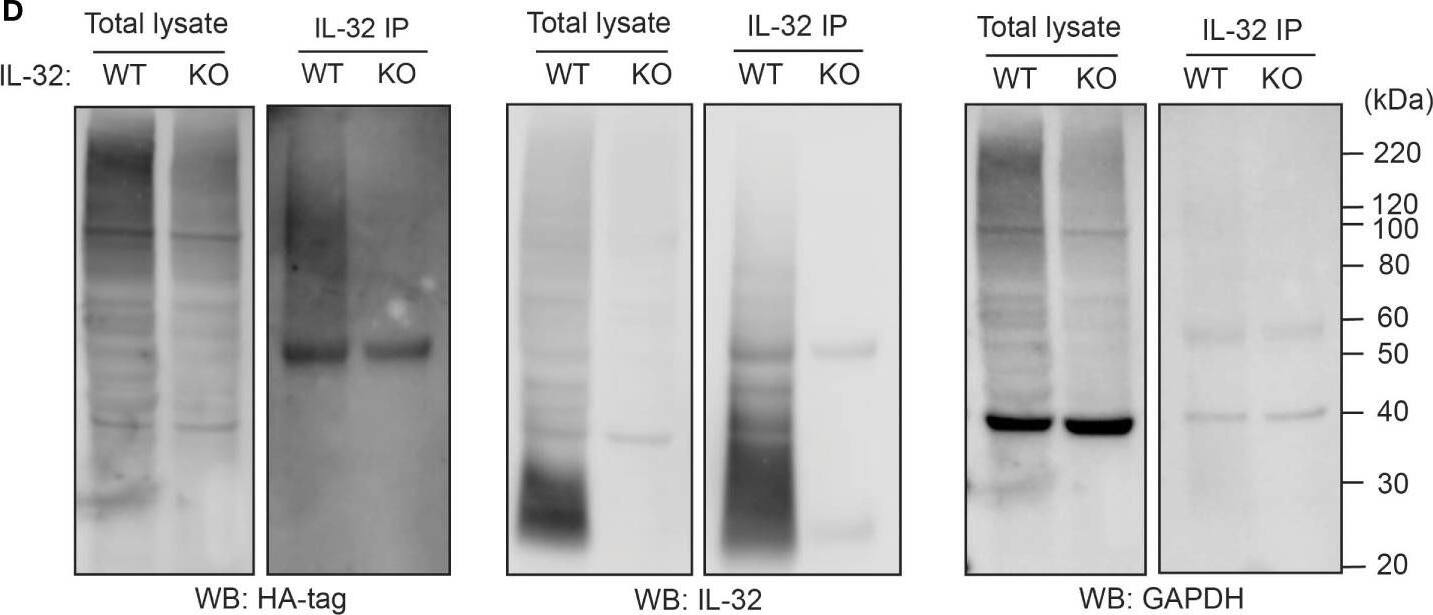
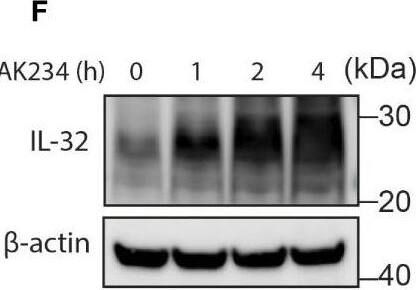
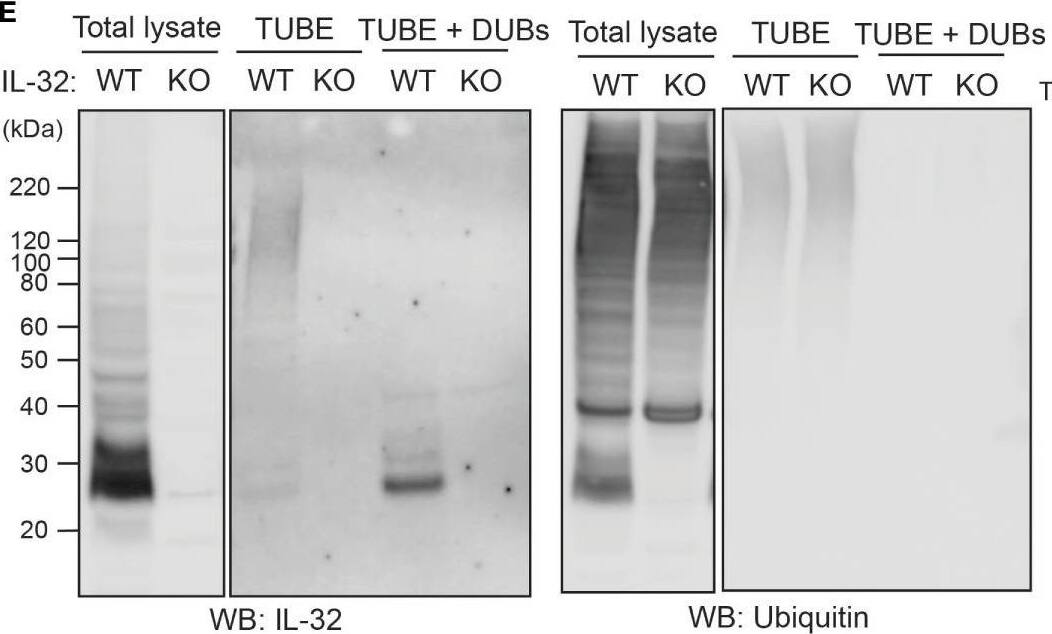
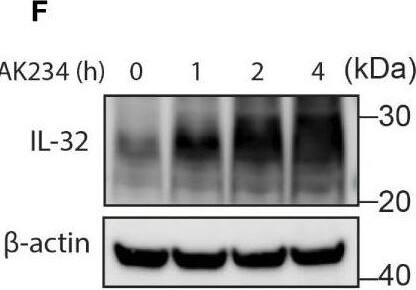
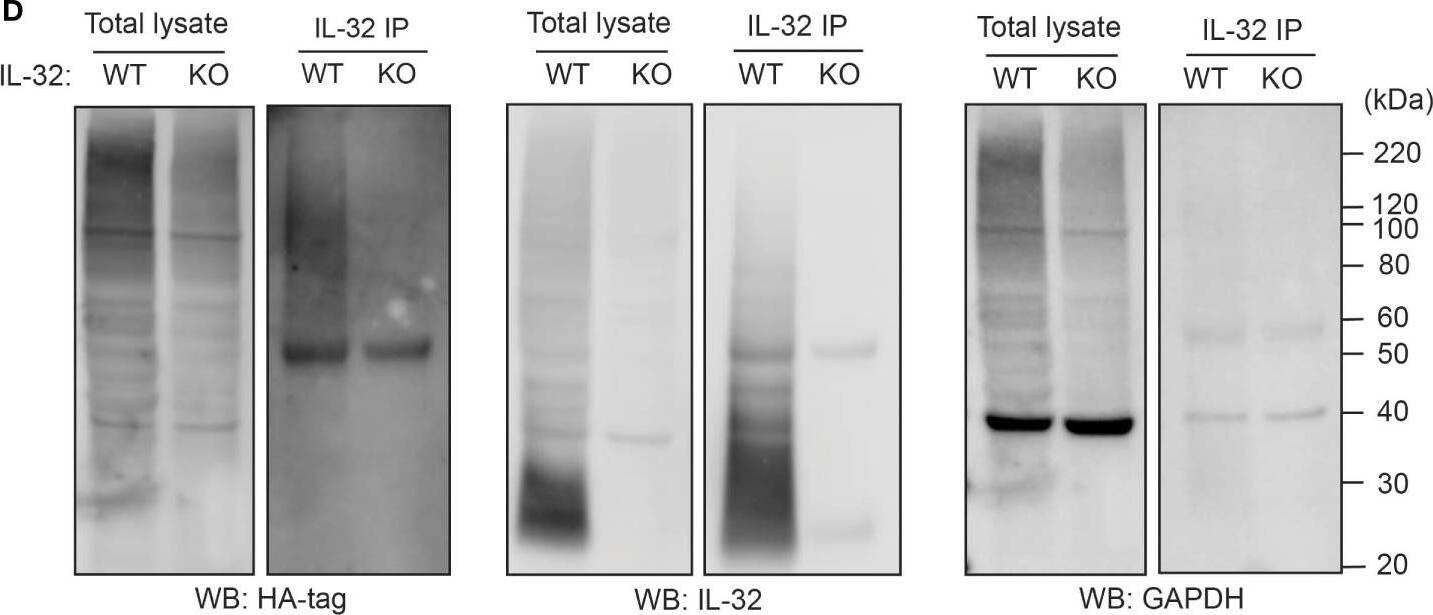
IL-32 is degraded through the ubiquitin-proteasome pathway. (A, B) JJN3 cells were treated with 20 µM MG-132 and harvested at the indicated time points. IL-32 protein levels were analyzed by (A) Western blotting, and (B) IL-32 mRNA was assessed by qPCR using GAPDH as housekeeping gene. The bars show the mean RQ of IL-32 ± SD. (C) Confocal images of JJN3 cells treated for 4 h with 100 nM carfilzomib and stained with IL-32 antibody (green) and Hoechst (blue). (D) JJN3 cells were transfected with HA-ubiquitin plasmid and incubated in hypoxia overnight before the cells were harvested and IL-32 immunoprecipitation was performed. Protein levels of HA-ubiquitin, IL-32, and GAPDH loading control were evaluated on WB. Total lysate and IP samples from the same membrane are shown with different brightness/contrast. (E) JJN3 cells were incubated overnight in hypoxia before they were stimulated with MG-132 for 4 h and harvested for TUBE assay of ubiquitinylated proteins. The presence of IL-32 and ubiquitin protein in TUBE pulldown was assessed by WB. DUB treatment for reversal of polyubiqutinylation was included to validate ubiquitin/TUBE pulldown. Total lysate and TUBE samples from the same membrane are shown with different brightness/contrast. (F) JJN3 cells were stimulated with 2 μM TAK234 before the cells were harvested, and IL-32 levels were analyzed by WB. (A–F) One representative experiment out of three is shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
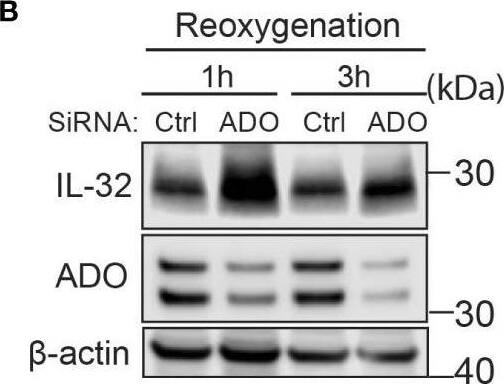
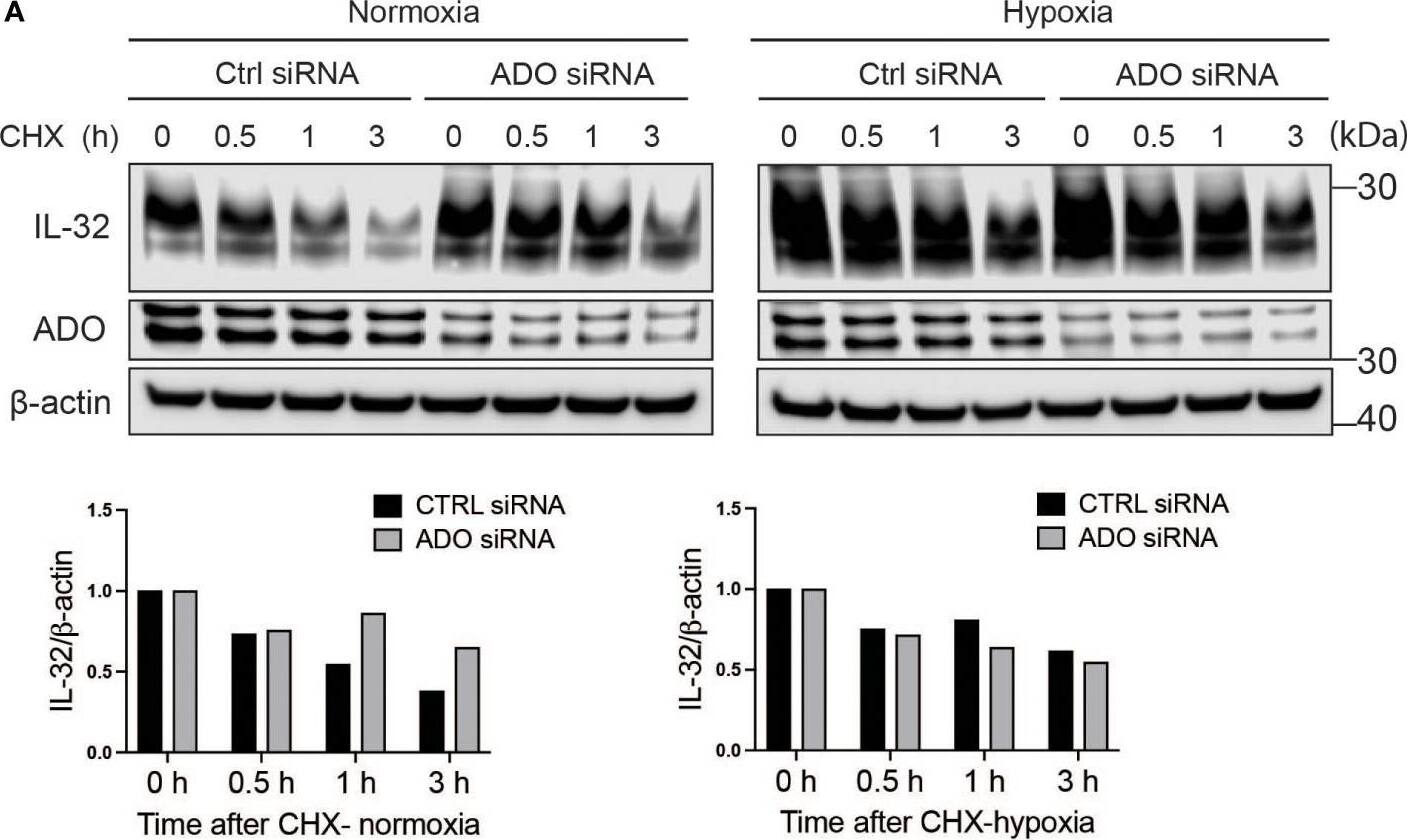
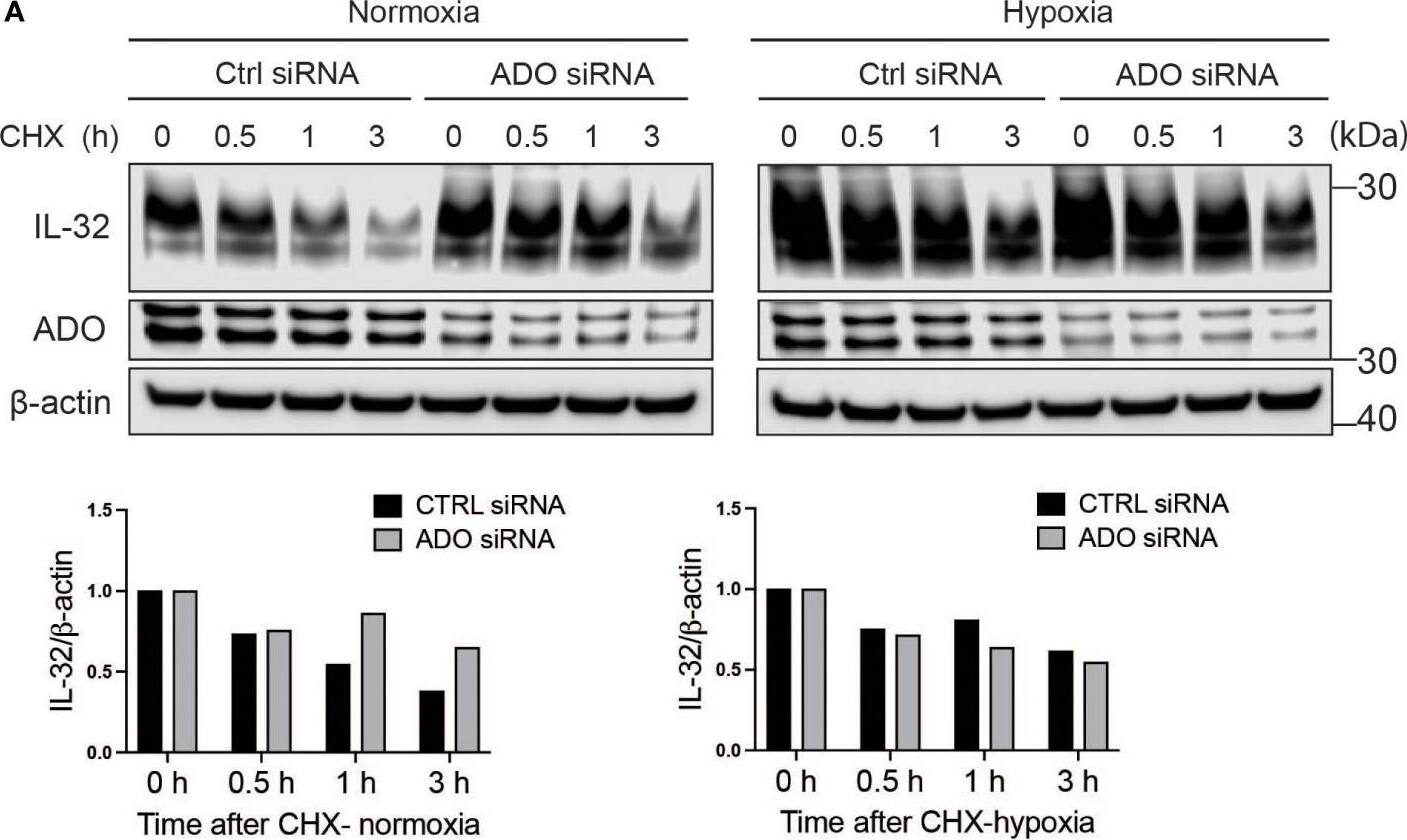
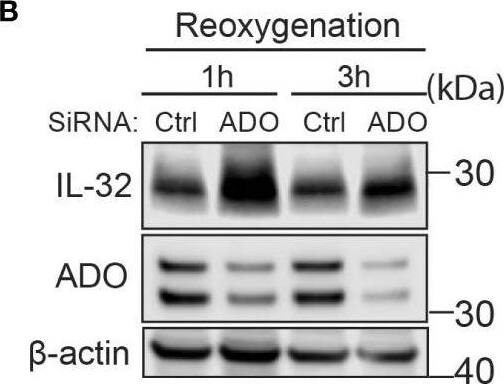
IL-32 protein half-life is regulated by the oxygen sensor ADO. (A) JJN-3 cells were transfected with ADO- and nontargeting Ctrl siRNA. After 24 h, the cells were seeded and cultured overnight in normoxia or hypoxia before being treated with 5 μg/ml CHX and the IL-32 CHX chase assay in normoxia and hypoxia. One representative WB of IL-32 and ADO siRNA-treated cells of n = 5 independent experiments is shown. (B) JJN-3 cells were transfected with ADO and nontargeting Ctrl siRNA. After being transfected for 24 h, the cells were cultured overnight in hypoxia before being treated with 5 µg/ml CHX and reoxygenized in normoxic culture conditions. Cells were harvested at indicated time points. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
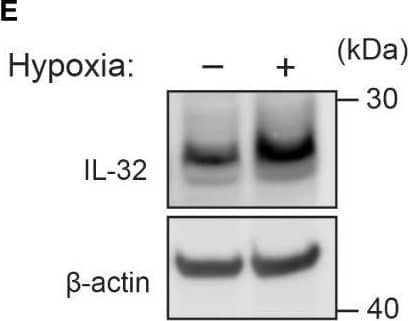
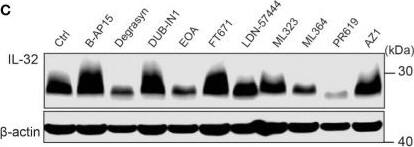
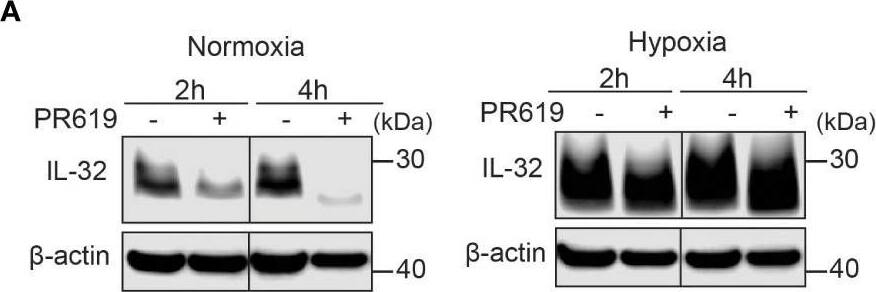
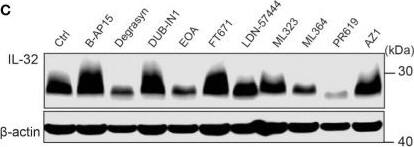
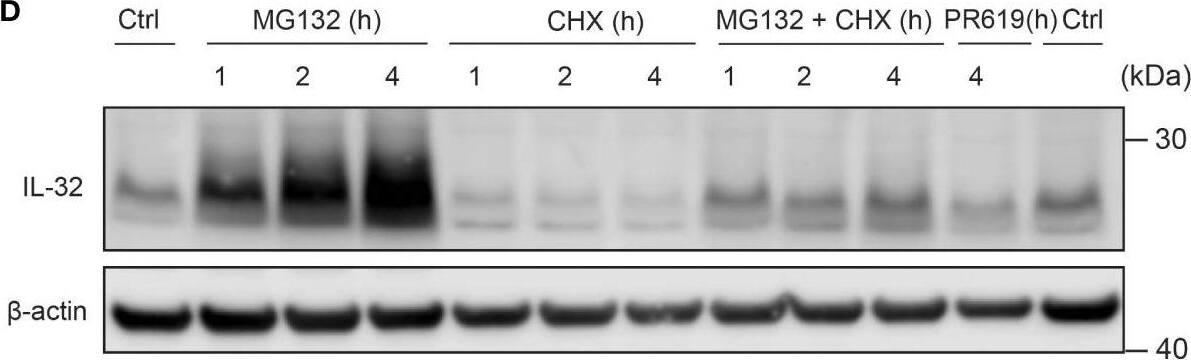
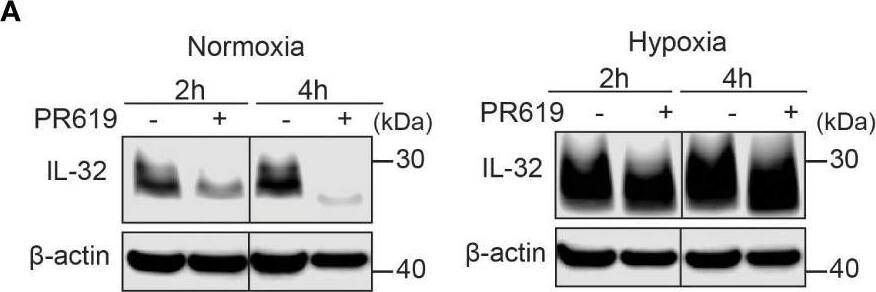
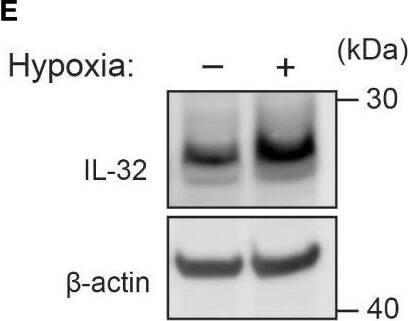
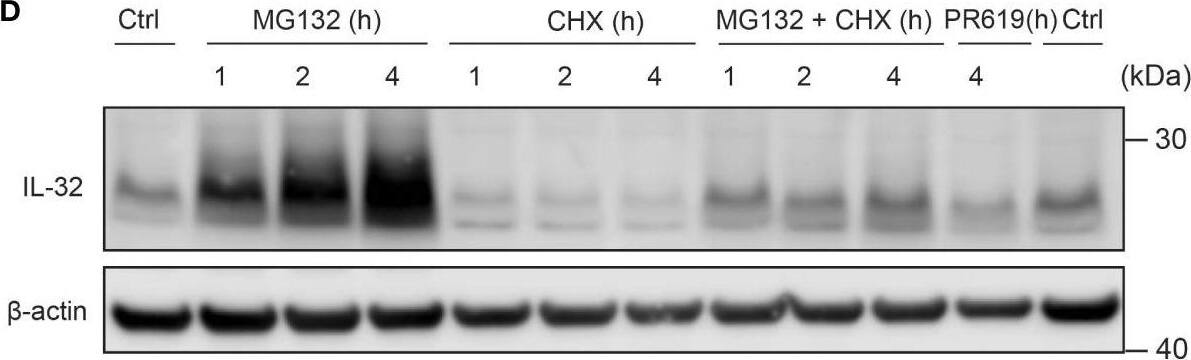
IL-32 is stabilized by deubiquitinases. (A) JJN3 cells were stimulated with PR619 (30 μM during normoxia and hypoxia before the sample was harvested at indicated time points. The figure shows representative WB of IL-32 protein levels of n = 3 independent experiments. (B) JJN3 cells were incubated overnight in hypoxia before they were moved to normoxia for 4 h with and without PR619 stimulation. Cells were harvested, and lysates were processed in the presence of a NEM-DUB inhibitor. Ubiqutinylated and nonubiqutinylated 27 kDA IL-32 proteins were assessed by WB. (C) JJN3 cells were stimulated with a panel of DUB inhibitors (see Supplementary Table S1 for concentrations) for 4 h, and IL-32 protein levels were analyzed by WB. Shown here is the representative WB of n = 3 independent experiments. (D) Human primary T cells isolated from healthy blood donors were treated with CHX (5 µg/ml), MG132 (20 µM), and PR619 (30 µM) and harvested at the indicated time points. Shown here is the representative WB from experiments with n = 3 donors. (E) Human primary T cells isolated from healthy blood donors were cultured overnight in hypoxia before IL-32 protein levels were assessed by WB. A representative WB from experiments with three different donors is shown here. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-32 by Western Blot
IL-32 is degraded through the ubiquitin-proteasome pathway. (A, B) JJN3 cells were treated with 20 µM MG-132 and harvested at the indicated time points. IL-32 protein levels were analyzed by (A) Western blotting, and (B) IL-32 mRNA was assessed by qPCR using GAPDH as housekeeping gene. The bars show the mean RQ of IL-32 ± SD. (C) Confocal images of JJN3 cells treated for 4 h with 100 nM carfilzomib and stained with IL-32 antibody (green) and Hoechst (blue). (D) JJN3 cells were transfected with HA-ubiquitin plasmid and incubated in hypoxia overnight before the cells were harvested and IL-32 immunoprecipitation was performed. Protein levels of HA-ubiquitin, IL-32, and GAPDH loading control were evaluated on WB. Total lysate and IP samples from the same membrane are shown with different brightness/contrast. (E) JJN3 cells were incubated overnight in hypoxia before they were stimulated with MG-132 for 4 h and harvested for TUBE assay of ubiquitinylated proteins. The presence of IL-32 and ubiquitin protein in TUBE pulldown was assessed by WB. DUB treatment for reversal of polyubiqutinylation was included to validate ubiquitin/TUBE pulldown. Total lysate and TUBE samples from the same membrane are shown with different brightness/contrast. (F) JJN3 cells were stimulated with 2 μM TAK234 before the cells were harvested, and IL-32 levels were analyzed by WB. (A–F) One representative experiment out of three is shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-32 by Western Blot
IL-32 is degraded through the ubiquitin-proteasome pathway. (A, B) JJN3 cells were treated with 20 µM MG-132 and harvested at the indicated time points. IL-32 protein levels were analyzed by (A) Western blotting, and (B) IL-32 mRNA was assessed by qPCR using GAPDH as housekeeping gene. The bars show the mean RQ of IL-32 ± SD. (C) Confocal images of JJN3 cells treated for 4 h with 100 nM carfilzomib and stained with IL-32 antibody (green) and Hoechst (blue). (D) JJN3 cells were transfected with HA-ubiquitin plasmid and incubated in hypoxia overnight before the cells were harvested and IL-32 immunoprecipitation was performed. Protein levels of HA-ubiquitin, IL-32, and GAPDH loading control were evaluated on WB. Total lysate and IP samples from the same membrane are shown with different brightness/contrast. (E) JJN3 cells were incubated overnight in hypoxia before they were stimulated with MG-132 for 4 h and harvested for TUBE assay of ubiquitinylated proteins. The presence of IL-32 and ubiquitin protein in TUBE pulldown was assessed by WB. DUB treatment for reversal of polyubiqutinylation was included to validate ubiquitin/TUBE pulldown. Total lysate and TUBE samples from the same membrane are shown with different brightness/contrast. (F) JJN3 cells were stimulated with 2 μM TAK234 before the cells were harvested, and IL-32 levels were analyzed by WB. (A–F) One representative experiment out of three is shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
IL-32 is stabilized by deubiquitinases. (A) JJN3 cells were stimulated with PR619 (30 μM during normoxia and hypoxia before the sample was harvested at indicated time points. The figure shows representative WB of IL-32 protein levels of n = 3 independent experiments. (B) JJN3 cells were incubated overnight in hypoxia before they were moved to normoxia for 4 h with and without PR619 stimulation. Cells were harvested, and lysates were processed in the presence of a NEM-DUB inhibitor. Ubiqutinylated and nonubiqutinylated 27 kDA IL-32 proteins were assessed by WB. (C) JJN3 cells were stimulated with a panel of DUB inhibitors (see Supplementary Table S1 for concentrations) for 4 h, and IL-32 protein levels were analyzed by WB. Shown here is the representative WB of n = 3 independent experiments. (D) Human primary T cells isolated from healthy blood donors were treated with CHX (5 µg/ml), MG132 (20 µM), and PR619 (30 µM) and harvested at the indicated time points. Shown here is the representative WB from experiments with n = 3 donors. (E) Human primary T cells isolated from healthy blood donors were cultured overnight in hypoxia before IL-32 protein levels were assessed by WB. A representative WB from experiments with three different donors is shown here. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
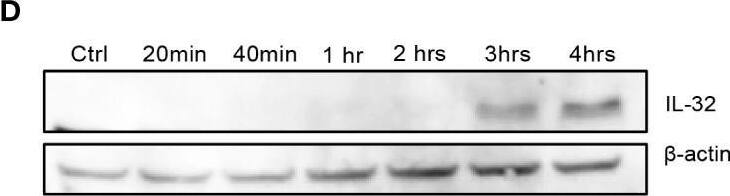
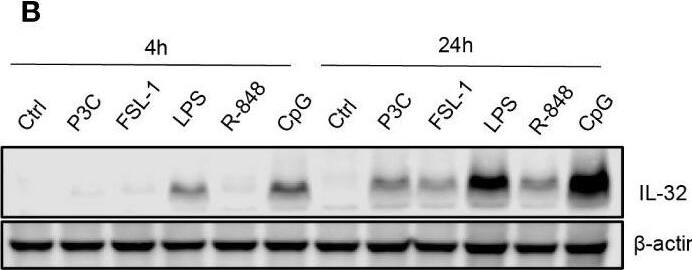
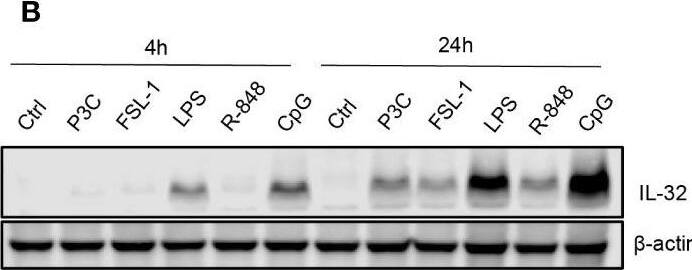
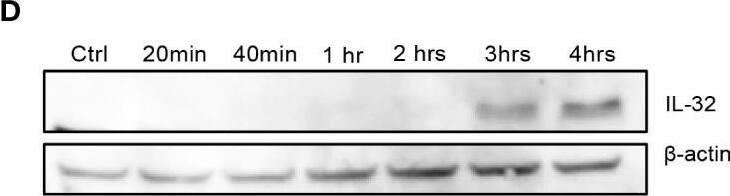
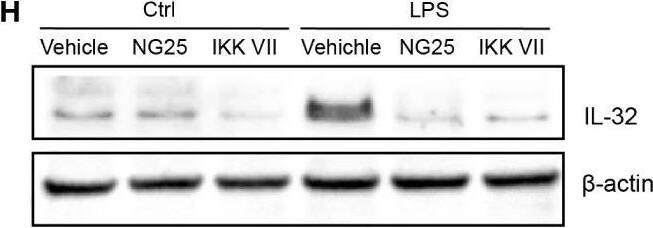
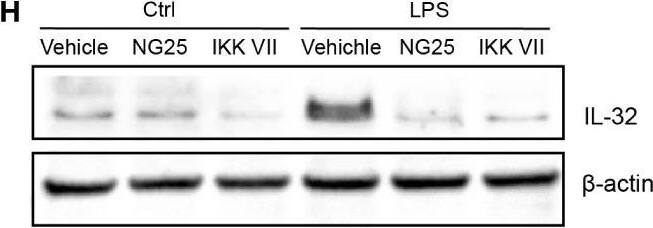
TLR-induced NF kappaB signaling promotes IL-32 expression in MM cells. (A) RPMI-8226 cells were stimulated with TLR agonists (for concentrations, see methods) for 4 and 24 hours and IL-32 mRNA expression was assessed by qPCR. The figure shows mean ± SEM of 3 independent experiments. (B) RPMI-8226 cells were stimulated with TLR agonists for 4 and 24 hours and IL-32 protein expression was evaluated by western blot. The figure shows representative western blot of 3 independent experiments. (C) RPMI-8226 cells were harvested at different time-points following LPS stimulation (0.1 µg/mL) and IL-32 mRNA expression was analyzed by qPCR (mean ± SD) and (D) IL-32 protein expression by western blot (E) RPMI-8226 TLR4 WT (mock) and KO cell lines were stimulated with LPS and CpG for 24 hours. Figure shows representative western blot (n=3) of IL-32 protein and qPCR analysis of IL-32 mRNA (mean ± SD, n=1) (F) RPMI-8226 TLR9 WT (mock) and KO cell lines were stimulated with LPS and CpG for 24 hours. The figure shows representative western blot (n=2) of IL-32 protein and qPCR analysis of IL-32 mRNA (mean ± SD, n=1) (G) RPMI-8226 cells were stimulated with LPS (0.1 µg/mL) and NG25 (2 µM) or IKK VII (10 µM) for 4 hours. IL-32 mRNA expression (mean ± SEM, n=3) was assessed by qPCR. (H) RPMI-8226 cells were stimulated with LPS, NG25 and IKK VII (concentrations as above) for 4 hours. The figure shows representative western blot (n=3) of IL-32 protein expression. P-values in (A) and (G) are calculated by one-way ANOVA with Dunnett´s multiple comparison test. *p≤ 0.05, **p ≤ 0.001, ****p ≤ 0.0001. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36875074), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
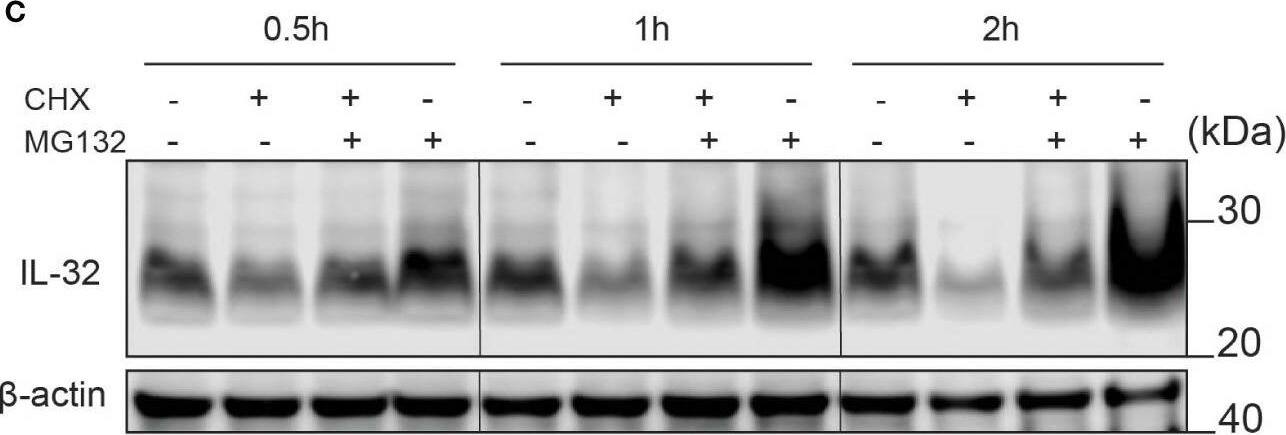
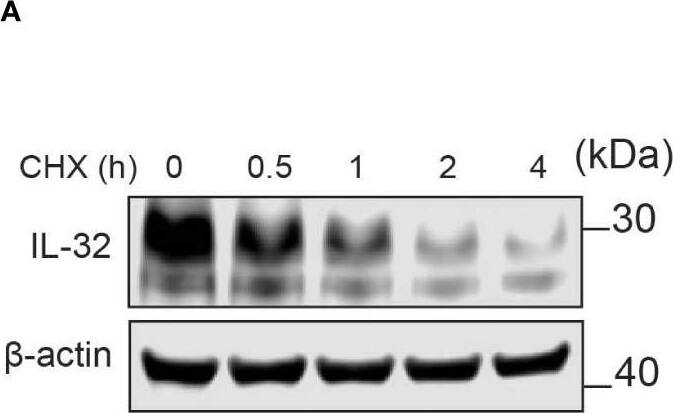
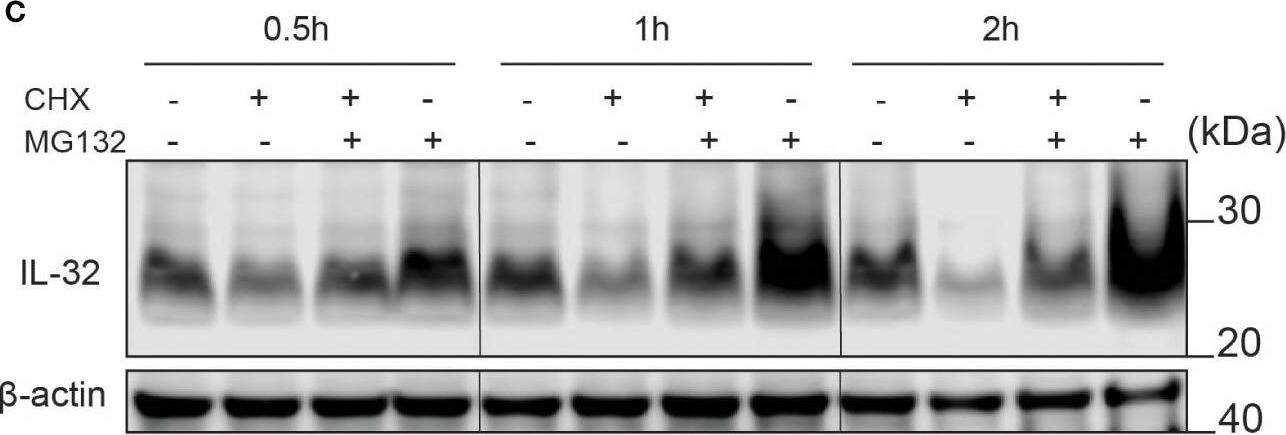
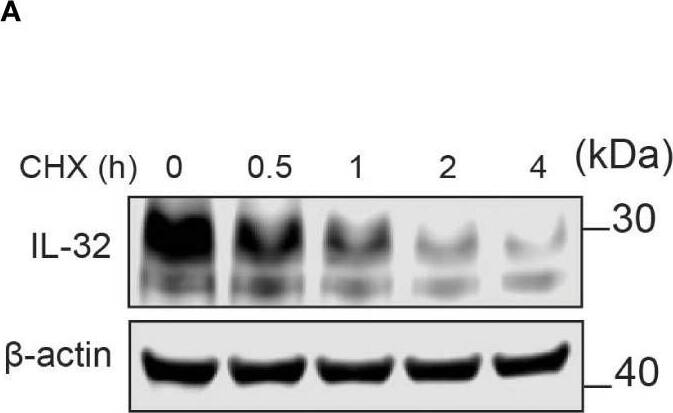
IL-32 has a high protein turnover. (A) JJN-3 cells were treated with CHX and harvested at the indicated time points. IL-32 protein levels were analyzed by WB. (B) Kinetics of IL-32 degradation in JJN3- cells. IL-32 protein signal intensity was quantified and normalized to loading control in n = 6 CHX chase experiments. The mean ±SEM is shown here. (C) JJN-3 cells were treated with 5 μg/ml CHX and 20 μM MG132 alone and in combination and harvested at the indicated time points. The figure shows one representative WB from n = 3 independent experiments. (D) Signal intensities of IL-32 and beta-actin were quantified from n = 3 independent experiments performed as in (C), and the values from treated samples at each time point were normalized relative to the control sample. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
TLR-induced NF kappaB signaling promotes IL-32 expression in MM cells. (A) RPMI-8226 cells were stimulated with TLR agonists (for concentrations, see methods) for 4 and 24 hours and IL-32 mRNA expression was assessed by qPCR. The figure shows mean ± SEM of 3 independent experiments. (B) RPMI-8226 cells were stimulated with TLR agonists for 4 and 24 hours and IL-32 protein expression was evaluated by western blot. The figure shows representative western blot of 3 independent experiments. (C) RPMI-8226 cells were harvested at different time-points following LPS stimulation (0.1 µg/mL) and IL-32 mRNA expression was analyzed by qPCR (mean ± SD) and (D) IL-32 protein expression by western blot (E) RPMI-8226 TLR4 WT (mock) and KO cell lines were stimulated with LPS and CpG for 24 hours. Figure shows representative western blot (n=3) of IL-32 protein and qPCR analysis of IL-32 mRNA (mean ± SD, n=1) (F) RPMI-8226 TLR9 WT (mock) and KO cell lines were stimulated with LPS and CpG for 24 hours. The figure shows representative western blot (n=2) of IL-32 protein and qPCR analysis of IL-32 mRNA (mean ± SD, n=1) (G) RPMI-8226 cells were stimulated with LPS (0.1 µg/mL) and NG25 (2 µM) or IKK VII (10 µM) for 4 hours. IL-32 mRNA expression (mean ± SEM, n=3) was assessed by qPCR. (H) RPMI-8226 cells were stimulated with LPS, NG25 and IKK VII (concentrations as above) for 4 hours. The figure shows representative western blot (n=3) of IL-32 protein expression. P-values in (A) and (G) are calculated by one-way ANOVA with Dunnett´s multiple comparison test. *p≤ 0.05, **p ≤ 0.001, ****p ≤ 0.0001. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36875074), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
TLR-induced NF kappaB signaling promotes IL-32 expression in MM cells. (A) RPMI-8226 cells were stimulated with TLR agonists (for concentrations, see methods) for 4 and 24 hours and IL-32 mRNA expression was assessed by qPCR. The figure shows mean ± SEM of 3 independent experiments. (B) RPMI-8226 cells were stimulated with TLR agonists for 4 and 24 hours and IL-32 protein expression was evaluated by western blot. The figure shows representative western blot of 3 independent experiments. (C) RPMI-8226 cells were harvested at different time-points following LPS stimulation (0.1 µg/mL) and IL-32 mRNA expression was analyzed by qPCR (mean ± SD) and (D) IL-32 protein expression by western blot (E) RPMI-8226 TLR4 WT (mock) and KO cell lines were stimulated with LPS and CpG for 24 hours. Figure shows representative western blot (n=3) of IL-32 protein and qPCR analysis of IL-32 mRNA (mean ± SD, n=1) (F) RPMI-8226 TLR9 WT (mock) and KO cell lines were stimulated with LPS and CpG for 24 hours. The figure shows representative western blot (n=2) of IL-32 protein and qPCR analysis of IL-32 mRNA (mean ± SD, n=1) (G) RPMI-8226 cells were stimulated with LPS (0.1 µg/mL) and NG25 (2 µM) or IKK VII (10 µM) for 4 hours. IL-32 mRNA expression (mean ± SEM, n=3) was assessed by qPCR. (H) RPMI-8226 cells were stimulated with LPS, NG25 and IKK VII (concentrations as above) for 4 hours. The figure shows representative western blot (n=3) of IL-32 protein expression. P-values in (A) and (G) are calculated by one-way ANOVA with Dunnett´s multiple comparison test. *p≤ 0.05, **p ≤ 0.001, ****p ≤ 0.0001. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36875074), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
IL-32 is stabilized by deubiquitinases. (A) JJN3 cells were stimulated with PR619 (30 μM during normoxia and hypoxia before the sample was harvested at indicated time points. The figure shows representative WB of IL-32 protein levels of n = 3 independent experiments. (B) JJN3 cells were incubated overnight in hypoxia before they were moved to normoxia for 4 h with and without PR619 stimulation. Cells were harvested, and lysates were processed in the presence of a NEM-DUB inhibitor. Ubiqutinylated and nonubiqutinylated 27 kDA IL-32 proteins were assessed by WB. (C) JJN3 cells were stimulated with a panel of DUB inhibitors (see Supplementary Table S1 for concentrations) for 4 h, and IL-32 protein levels were analyzed by WB. Shown here is the representative WB of n = 3 independent experiments. (D) Human primary T cells isolated from healthy blood donors were treated with CHX (5 µg/ml), MG132 (20 µM), and PR619 (30 µM) and harvested at the indicated time points. Shown here is the representative WB from experiments with n = 3 donors. (E) Human primary T cells isolated from healthy blood donors were cultured overnight in hypoxia before IL-32 protein levels were assessed by WB. A representative WB from experiments with three different donors is shown here. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
IL-32 has a high protein turnover. (A) JJN-3 cells were treated with CHX and harvested at the indicated time points. IL-32 protein levels were analyzed by WB. (B) Kinetics of IL-32 degradation in JJN3- cells. IL-32 protein signal intensity was quantified and normalized to loading control in n = 6 CHX chase experiments. The mean ±SEM is shown here. (C) JJN-3 cells were treated with 5 μg/ml CHX and 20 μM MG132 alone and in combination and harvested at the indicated time points. The figure shows one representative WB from n = 3 independent experiments. (D) Signal intensities of IL-32 and beta-actin were quantified from n = 3 independent experiments performed as in (C), and the values from treated samples at each time point were normalized relative to the control sample. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
IL-32 protein half-life is regulated by the oxygen sensor ADO. (A) JJN-3 cells were transfected with ADO- and nontargeting Ctrl siRNA. After 24 h, the cells were seeded and cultured overnight in normoxia or hypoxia before being treated with 5 μg/ml CHX and the IL-32 CHX chase assay in normoxia and hypoxia. One representative WB of IL-32 and ADO siRNA-treated cells of n = 5 independent experiments is shown. (B) JJN-3 cells were transfected with ADO and nontargeting Ctrl siRNA. After being transfected for 24 h, the cells were cultured overnight in hypoxia before being treated with 5 µg/ml CHX and reoxygenized in normoxic culture conditions. Cells were harvested at indicated time points. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
IL-32 is stabilized by deubiquitinases. (A) JJN3 cells were stimulated with PR619 (30 μM during normoxia and hypoxia before the sample was harvested at indicated time points. The figure shows representative WB of IL-32 protein levels of n = 3 independent experiments. (B) JJN3 cells were incubated overnight in hypoxia before they were moved to normoxia for 4 h with and without PR619 stimulation. Cells were harvested, and lysates were processed in the presence of a NEM-DUB inhibitor. Ubiqutinylated and nonubiqutinylated 27 kDA IL-32 proteins were assessed by WB. (C) JJN3 cells were stimulated with a panel of DUB inhibitors (see Supplementary Table S1 for concentrations) for 4 h, and IL-32 protein levels were analyzed by WB. Shown here is the representative WB of n = 3 independent experiments. (D) Human primary T cells isolated from healthy blood donors were treated with CHX (5 µg/ml), MG132 (20 µM), and PR619 (30 µM) and harvested at the indicated time points. Shown here is the representative WB from experiments with n = 3 donors. (E) Human primary T cells isolated from healthy blood donors were cultured overnight in hypoxia before IL-32 protein levels were assessed by WB. A representative WB from experiments with three different donors is shown here. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
IL-32 has a high protein turnover. (A) JJN-3 cells were treated with CHX and harvested at the indicated time points. IL-32 protein levels were analyzed by WB. (B) Kinetics of IL-32 degradation in JJN3- cells. IL-32 protein signal intensity was quantified and normalized to loading control in n = 6 CHX chase experiments. The mean ±SEM is shown here. (C) JJN-3 cells were treated with 5 μg/ml CHX and 20 μM MG132 alone and in combination and harvested at the indicated time points. The figure shows one representative WB from n = 3 independent experiments. (D) Signal intensities of IL-32 and beta-actin were quantified from n = 3 independent experiments performed as in (C), and the values from treated samples at each time point were normalized relative to the control sample. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-32 by Western Blot
IL-32 is degraded through the ubiquitin-proteasome pathway. (A, B) JJN3 cells were treated with 20 µM MG-132 and harvested at the indicated time points. IL-32 protein levels were analyzed by (A) Western blotting, and (B) IL-32 mRNA was assessed by qPCR using GAPDH as housekeeping gene. The bars show the mean RQ of IL-32 ± SD. (C) Confocal images of JJN3 cells treated for 4 h with 100 nM carfilzomib and stained with IL-32 antibody (green) and Hoechst (blue). (D) JJN3 cells were transfected with HA-ubiquitin plasmid and incubated in hypoxia overnight before the cells were harvested and IL-32 immunoprecipitation was performed. Protein levels of HA-ubiquitin, IL-32, and GAPDH loading control were evaluated on WB. Total lysate and IP samples from the same membrane are shown with different brightness/contrast. (E) JJN3 cells were incubated overnight in hypoxia before they were stimulated with MG-132 for 4 h and harvested for TUBE assay of ubiquitinylated proteins. The presence of IL-32 and ubiquitin protein in TUBE pulldown was assessed by WB. DUB treatment for reversal of polyubiqutinylation was included to validate ubiquitin/TUBE pulldown. Total lysate and TUBE samples from the same membrane are shown with different brightness/contrast. (F) JJN3 cells were stimulated with 2 μM TAK234 before the cells were harvested, and IL-32 levels were analyzed by WB. (A–F) One representative experiment out of three is shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
IL-32 has a high protein turnover. (A) JJN-3 cells were treated with CHX and harvested at the indicated time points. IL-32 protein levels were analyzed by WB. (B) Kinetics of IL-32 degradation in JJN3- cells. IL-32 protein signal intensity was quantified and normalized to loading control in n = 6 CHX chase experiments. The mean ±SEM is shown here. (C) JJN-3 cells were treated with 5 μg/ml CHX and 20 μM MG132 alone and in combination and harvested at the indicated time points. The figure shows one representative WB from n = 3 independent experiments. (D) Signal intensities of IL-32 and beta-actin were quantified from n = 3 independent experiments performed as in (C), and the values from treated samples at each time point were normalized relative to the control sample. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-32 by Western Blot
IL-32 is degraded through the ubiquitin-proteasome pathway. (A, B) JJN3 cells were treated with 20 µM MG-132 and harvested at the indicated time points. IL-32 protein levels were analyzed by (A) Western blotting, and (B) IL-32 mRNA was assessed by qPCR using GAPDH as housekeeping gene. The bars show the mean RQ of IL-32 ± SD. (C) Confocal images of JJN3 cells treated for 4 h with 100 nM carfilzomib and stained with IL-32 antibody (green) and Hoechst (blue). (D) JJN3 cells were transfected with HA-ubiquitin plasmid and incubated in hypoxia overnight before the cells were harvested and IL-32 immunoprecipitation was performed. Protein levels of HA-ubiquitin, IL-32, and GAPDH loading control were evaluated on WB. Total lysate and IP samples from the same membrane are shown with different brightness/contrast. (E) JJN3 cells were incubated overnight in hypoxia before they were stimulated with MG-132 for 4 h and harvested for TUBE assay of ubiquitinylated proteins. The presence of IL-32 and ubiquitin protein in TUBE pulldown was assessed by WB. DUB treatment for reversal of polyubiqutinylation was included to validate ubiquitin/TUBE pulldown. Total lysate and TUBE samples from the same membrane are shown with different brightness/contrast. (F) JJN3 cells were stimulated with 2 μM TAK234 before the cells were harvested, and IL-32 levels were analyzed by WB. (A–F) One representative experiment out of three is shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
TLR-induced NF kappaB signaling promotes IL-32 expression in MM cells. (A) RPMI-8226 cells were stimulated with TLR agonists (for concentrations, see methods) for 4 and 24 hours and IL-32 mRNA expression was assessed by qPCR. The figure shows mean ± SEM of 3 independent experiments. (B) RPMI-8226 cells were stimulated with TLR agonists for 4 and 24 hours and IL-32 protein expression was evaluated by western blot. The figure shows representative western blot of 3 independent experiments. (C) RPMI-8226 cells were harvested at different time-points following LPS stimulation (0.1 µg/mL) and IL-32 mRNA expression was analyzed by qPCR (mean ± SD) and (D) IL-32 protein expression by western blot (E) RPMI-8226 TLR4 WT (mock) and KO cell lines were stimulated with LPS and CpG for 24 hours. Figure shows representative western blot (n=3) of IL-32 protein and qPCR analysis of IL-32 mRNA (mean ± SD, n=1) (F) RPMI-8226 TLR9 WT (mock) and KO cell lines were stimulated with LPS and CpG for 24 hours. The figure shows representative western blot (n=2) of IL-32 protein and qPCR analysis of IL-32 mRNA (mean ± SD, n=1) (G) RPMI-8226 cells were stimulated with LPS (0.1 µg/mL) and NG25 (2 µM) or IKK VII (10 µM) for 4 hours. IL-32 mRNA expression (mean ± SEM, n=3) was assessed by qPCR. (H) RPMI-8226 cells were stimulated with LPS, NG25 and IKK VII (concentrations as above) for 4 hours. The figure shows representative western blot (n=3) of IL-32 protein expression. P-values in (A) and (G) are calculated by one-way ANOVA with Dunnett´s multiple comparison test. *p≤ 0.05, **p ≤ 0.001, ****p ≤ 0.0001. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36875074), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of Human IL-32 by Western Blot
IL-32 is degraded through the ubiquitin-proteasome pathway. (A, B) JJN3 cells were treated with 20 µM MG-132 and harvested at the indicated time points. IL-32 protein levels were analyzed by (A) Western blotting, and (B) IL-32 mRNA was assessed by qPCR using GAPDH as housekeeping gene. The bars show the mean RQ of IL-32 ± SD. (C) Confocal images of JJN3 cells treated for 4 h with 100 nM carfilzomib and stained with IL-32 antibody (green) and Hoechst (blue). (D) JJN3 cells were transfected with HA-ubiquitin plasmid and incubated in hypoxia overnight before the cells were harvested and IL-32 immunoprecipitation was performed. Protein levels of HA-ubiquitin, IL-32, and GAPDH loading control were evaluated on WB. Total lysate and IP samples from the same membrane are shown with different brightness/contrast. (E) JJN3 cells were incubated overnight in hypoxia before they were stimulated with MG-132 for 4 h and harvested for TUBE assay of ubiquitinylated proteins. The presence of IL-32 and ubiquitin protein in TUBE pulldown was assessed by WB. DUB treatment for reversal of polyubiqutinylation was included to validate ubiquitin/TUBE pulldown. Total lysate and TUBE samples from the same membrane are shown with different brightness/contrast. (F) JJN3 cells were stimulated with 2 μM TAK234 before the cells were harvested, and IL-32 levels were analyzed by WB. (A–F) One representative experiment out of three is shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
IL-32 is stabilized by deubiquitinases. (A) JJN3 cells were stimulated with PR619 (30 μM during normoxia and hypoxia before the sample was harvested at indicated time points. The figure shows representative WB of IL-32 protein levels of n = 3 independent experiments. (B) JJN3 cells were incubated overnight in hypoxia before they were moved to normoxia for 4 h with and without PR619 stimulation. Cells were harvested, and lysates were processed in the presence of a NEM-DUB inhibitor. Ubiqutinylated and nonubiqutinylated 27 kDA IL-32 proteins were assessed by WB. (C) JJN3 cells were stimulated with a panel of DUB inhibitors (see Supplementary Table S1 for concentrations) for 4 h, and IL-32 protein levels were analyzed by WB. Shown here is the representative WB of n = 3 independent experiments. (D) Human primary T cells isolated from healthy blood donors were treated with CHX (5 µg/ml), MG132 (20 µM), and PR619 (30 µM) and harvested at the indicated time points. Shown here is the representative WB from experiments with n = 3 donors. (E) Human primary T cells isolated from healthy blood donors were cultured overnight in hypoxia before IL-32 protein levels were assessed by WB. A representative WB from experiments with three different donors is shown here. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
IL-32 protein half-life is regulated by the oxygen sensor ADO. (A) JJN-3 cells were transfected with ADO- and nontargeting Ctrl siRNA. After 24 h, the cells were seeded and cultured overnight in normoxia or hypoxia before being treated with 5 μg/ml CHX and the IL-32 CHX chase assay in normoxia and hypoxia. One representative WB of IL-32 and ADO siRNA-treated cells of n = 5 independent experiments is shown. (B) JJN-3 cells were transfected with ADO and nontargeting Ctrl siRNA. After being transfected for 24 h, the cells were cultured overnight in hypoxia before being treated with 5 µg/ml CHX and reoxygenized in normoxic culture conditions. Cells were harvested at indicated time points. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
TLR-induced NF kappaB signaling promotes IL-32 expression in MM cells. (A) RPMI-8226 cells were stimulated with TLR agonists (for concentrations, see methods) for 4 and 24 hours and IL-32 mRNA expression was assessed by qPCR. The figure shows mean ± SEM of 3 independent experiments. (B) RPMI-8226 cells were stimulated with TLR agonists for 4 and 24 hours and IL-32 protein expression was evaluated by western blot. The figure shows representative western blot of 3 independent experiments. (C) RPMI-8226 cells were harvested at different time-points following LPS stimulation (0.1 µg/mL) and IL-32 mRNA expression was analyzed by qPCR (mean ± SD) and (D) IL-32 protein expression by western blot (E) RPMI-8226 TLR4 WT (mock) and KO cell lines were stimulated with LPS and CpG for 24 hours. Figure shows representative western blot (n=3) of IL-32 protein and qPCR analysis of IL-32 mRNA (mean ± SD, n=1) (F) RPMI-8226 TLR9 WT (mock) and KO cell lines were stimulated with LPS and CpG for 24 hours. The figure shows representative western blot (n=2) of IL-32 protein and qPCR analysis of IL-32 mRNA (mean ± SD, n=1) (G) RPMI-8226 cells were stimulated with LPS (0.1 µg/mL) and NG25 (2 µM) or IKK VII (10 µM) for 4 hours. IL-32 mRNA expression (mean ± SEM, n=3) was assessed by qPCR. (H) RPMI-8226 cells were stimulated with LPS, NG25 and IKK VII (concentrations as above) for 4 hours. The figure shows representative western blot (n=3) of IL-32 protein expression. P-values in (A) and (G) are calculated by one-way ANOVA with Dunnett´s multiple comparison test. *p≤ 0.05, **p ≤ 0.001, ****p ≤ 0.0001. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36875074), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
IL-32 is stabilized by deubiquitinases. (A) JJN3 cells were stimulated with PR619 (30 μM during normoxia and hypoxia before the sample was harvested at indicated time points. The figure shows representative WB of IL-32 protein levels of n = 3 independent experiments. (B) JJN3 cells were incubated overnight in hypoxia before they were moved to normoxia for 4 h with and without PR619 stimulation. Cells were harvested, and lysates were processed in the presence of a NEM-DUB inhibitor. Ubiqutinylated and nonubiqutinylated 27 kDA IL-32 proteins were assessed by WB. (C) JJN3 cells were stimulated with a panel of DUB inhibitors (see Supplementary Table S1 for concentrations) for 4 h, and IL-32 protein levels were analyzed by WB. Shown here is the representative WB of n = 3 independent experiments. (D) Human primary T cells isolated from healthy blood donors were treated with CHX (5 µg/ml), MG132 (20 µM), and PR619 (30 µM) and harvested at the indicated time points. Shown here is the representative WB from experiments with n = 3 donors. (E) Human primary T cells isolated from healthy blood donors were cultured overnight in hypoxia before IL-32 protein levels were assessed by WB. A representative WB from experiments with three different donors is shown here. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of IL-32 by Western Blot
IL-32 is stabilized by deubiquitinases. (A) JJN3 cells were stimulated with PR619 (30 μM during normoxia and hypoxia before the sample was harvested at indicated time points. The figure shows representative WB of IL-32 protein levels of n = 3 independent experiments. (B) JJN3 cells were incubated overnight in hypoxia before they were moved to normoxia for 4 h with and without PR619 stimulation. Cells were harvested, and lysates were processed in the presence of a NEM-DUB inhibitor. Ubiqutinylated and nonubiqutinylated 27 kDA IL-32 proteins were assessed by WB. (C) JJN3 cells were stimulated with a panel of DUB inhibitors (see Supplementary Table S1 for concentrations) for 4 h, and IL-32 protein levels were analyzed by WB. Shown here is the representative WB of n = 3 independent experiments. (D) Human primary T cells isolated from healthy blood donors were treated with CHX (5 µg/ml), MG132 (20 µM), and PR619 (30 µM) and harvested at the indicated time points. Shown here is the representative WB from experiments with n = 3 donors. (E) Human primary T cells isolated from healthy blood donors were cultured overnight in hypoxia before IL-32 protein levels were assessed by WB. A representative WB from experiments with three different donors is shown here. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37313466), licensed under a CC-BY license. Not internally tested by R&D Systems.