Recombinant Human IL-3 GMP Protein, CF GMP
R&D Systems, part of Bio-Techne | Catalog # BT-003-GMP

Key Product Details
- IL-3 Manufactured in Bio-Techne's new GMP facility
- Lot-to-lot consistency
- Stringent guidelines for patient safety
- Scalability necessary to support successful therapeutics
- Learn more about manufacturing in our new GMP facility
- Test it in your process! Request a sample of GMP IL-3
Product Specifications
Source
Ala20 - Phe152, with and without an N-terminal Met
Produced using non-animal reagents in an animal-free laboratory.Manufactured and tested under cGMP guidelines.
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
SDS-PAGE
Activity
The ED50 for this effect is 0.0150-0.150 ng/mL.
Host Cell Protein
Mycoplasma
Host Cell DNA
Scientific Data Images for Recombinant Human IL-3 GMP Protein, CF
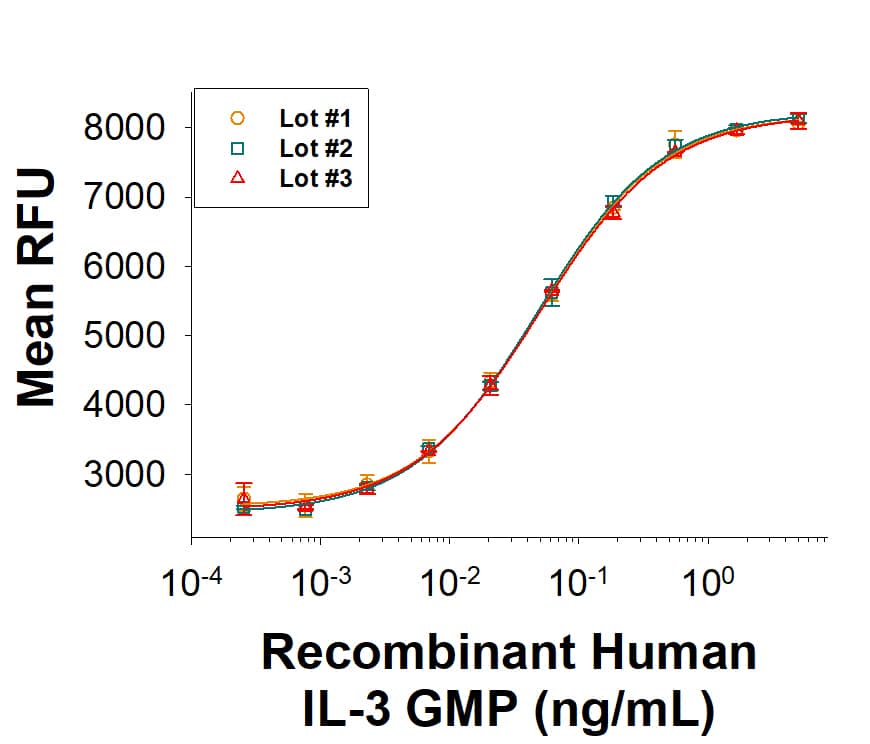
Recombinant Human IL‑3 GMP Protein Bioactivity.
The bioactivity of Recombinant Human IL-3 GMP Protein (Catalog # BT-003-GMP) was measured in a cell proliferation assay using TF‑1 human erythroleukemic cells. Three independent lots were tested for bioactivity and plotted on the same graph to show lot-to-lot consistency of GMPIL-3 protein.Equivalent Bioactivity of GMP and Animal-Free grades of Recombinant Human IL-3.
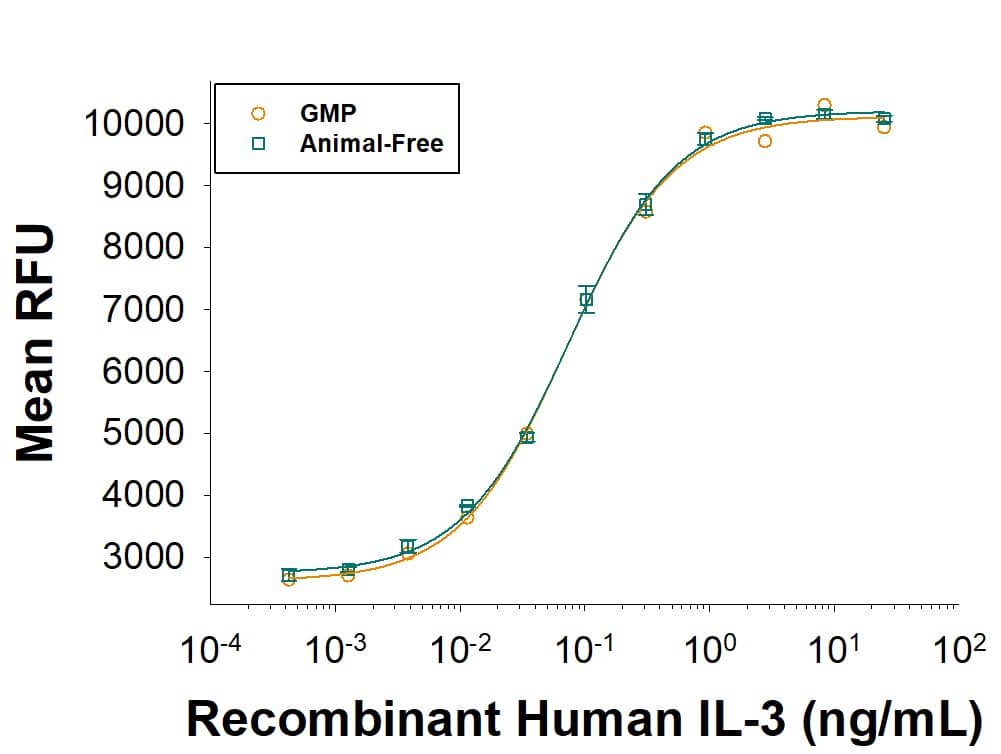
Equivalent bioactivity of GMP (Catalog # BT-003-GMP) and Animal-Free (BT-003-AFL) grades of Recombinant Human IL-3 as measured in a cell proliferation assay using TF‑1 human erythroleukemic cells (orange and green, respectively).Recombinant Human IL‑3 GMP Protein SDS-PAGE.
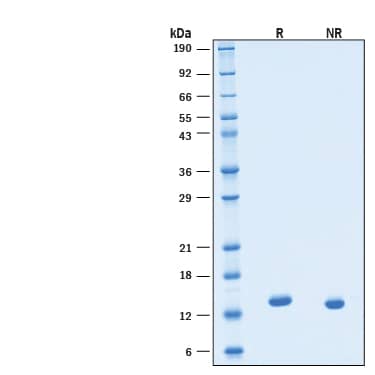
2 μg/lane of Recombinant Human IL‑3 GMP Protein (Catalog # BT-003-GMP) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing a band at 14 kDa under reducing conditions.Formulation, Preparation and Storage
BT-003-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in sterile water. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: IL-3
Interleukin 3 is a pleiotropic factor produced primarily by activated T cells that can stimulate the proliferation and differentiation of pluripotent hematopoietic stem cells as well as various lineage committed progenitors. In addition, IL-3 also affects the functional activity of mature mast cells, basophils, eosinophils and macrophages. Because of its multiple functions and targets, it was originally studied under different names, including mast cell growth factor, P-cell stimulating factor, burst promoting activity, multi-colony stimulating factor, thy-1 inducing factor and WEHI-3 growth factor. In addition to activated T cells, other cell types such as human thymic epithelial cells, activated murine mast cells, murine keratinocytes and neurons/astrocytes can also produce IL-3. At the amino acid sequence level, mature human and murine IL-3 share only 29% sequence identity. Consistent with this lack of homology, IL-3 activity is highly species-specific and human IL-3 does not show activity on murine cells.
IL-3 exerts its biological activities through binding to specific cell surface receptors. The high affinity receptor responsible for IL-3 signaling is composed of at least two subunits, an IL-3 specific alpha chain which binds IL-3 with low affinity and a common beta chain that is shared by the IL-5 and GM-CSF high-affinity receptors. Although the beta chain itself does not bind IL-3, it confers high-affinity IL-3 binding in the presence of the alpha chain. Receptors for IL-3 are present on bone marrow progenitors, macrophages, mast cells, eosinophils, megakaryocytes, basophils and various myeloid leukemic cells.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional IL-3 Products
Product Documents for Recombinant Human IL-3 GMP Protein, CF
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Designed, manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented and controlled manufacturing process
- Control of documentation and process changes by QA
- Personnel training programs
- Raw material inspection and vendor qualification/monitoring program
- Validated equipment, processes and test methods
- Equipment calibration and maintenance schedules using a Regulatory Asset Manager
- Facility/Utilities maintenance, contamination controls, safety and pest control programs
- Material review process for variances
- Robust product stability program following relevant ICH guidelines
- N-terminal amino acid analysis
- SDS-PAGE purity analysis
- Molecular weight analysis via mass spectrometry
- Endotoxin assessment per USP <85> and Ph. Eur. 2.6.14 guidelines
- Bioassay analysis
- Microbial testing per USP <71> and Ph. Eur. 2.6.1 guidelines
- Host cell protein assessment
- Host cell DNA assessment
- Mycoplasma assessment
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis and St. Paul, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Animal-Free Manufacturing Conditions
Our dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers.
Product Specific Notices for Recombinant Human IL-3 GMP Protein, CF
Full terms and conditions of sale can be found online in the Protein Sciences Segment T&Cs at: Terms & Conditions.
For preclinical, or clinical ex vivo use