Recombinant Human GDNF GMP Protein, CF GMP Best Seller
R&D Systems, part of Bio-Techne | Catalog # 212-GMP

Key Product Details
Source
Accession #
Structure / Form
Conjugate
Applications
Product Specifications
Source
Arg109-Ile211
Manufactured and tested under cGMP guidelines.
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
Activity
The ED50 for this effect is 2-12 ng/mL in the presence of Recombinant Human GFR alpha‑1/GDNF R alpha‑1 Fc Chimera (Catalog # 714-GR).
The specific activity of recombinant human GDNF is >5.0 x 105 units/mg, which is calibrated against the human GDNF Reference Standard (NIBSC code: 09/266).
Measured by its binding ability in a functional ELISA.
Immobilized Recombinant Human GFR alpha‑1/GDNF R alpha‑1 Fc Chimera (Catalog # 714-GR) at 1 µg/mL can bind Recombinant Human GDNF with an apparent Kd <1 nM.
Mycoplasma
Host Cell DNA
Scientific Data Images for Recombinant Human GDNF GMP Protein, CF
Recombinant Human GDNF GMP Protein Bioactivity
GMP-grade Recombinant Human GDNF (Catalog # 212-GMP) stimulates proliferation in the SH-SY5Y human neuroblastoma cell line. The ED50 for this effect is 2-12 ng/mL in the presence of Recombinant Human GFRa-1/GDNF Ra-1 Fc Chimera (714-GR).Recombinant Human GDNF GMP Protein SDS-PAGE
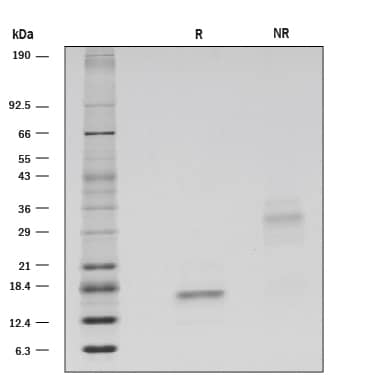
1 μg/lane of GMP-grade Recombinant Human GDNF (Catalog # 212-GMP) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by silver staining, showing bands at 17 kDa and 33 kDa, respectively.Formulation, Preparation and Storage
212-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution | Reconstitute at 100 μg/mL in PBS. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: GDNF
Native GDNF, a disulfide-linked homodimeric glycoprotein, is a novel member of the TGF-beta superfamily. Human GDNF cDNA encodes a 211 amino acid residue prepropeptide that is processed to yield a dimeric protein. Mature human GDNF was predicted to contain two 134 amino acid residue subunits. NS0 expressed mature human GDNF lacks 31 residues from the amino-terminus of the predicted sequence. This glycosylated recombinant mature human GDNF still contains the seven conserved Cys residues found in all members of the TGF-beta superfamily and is biologically active. The GDNF sequence contains two potential glycosylation sites and insect cell‑expressed recombinant rat GDNF proteins are glycosylated. Mature rat and human GDNF exhibit approximately 93% amino acid sequence identity and show considerable species cross-reactivity. Cells known to express GDNF include Sertoli cells, type 1 astrocytes, Schwann cells, neurons, pinealocytes and skeletal muscle cells.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional GDNF Products
Product Documents for Recombinant Human GDNF GMP Protein, CF
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Designed, manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented and controlled manufacturing processes
- Control of documentation and process changes by QA
- Personnel training programs
- Raw material inspection and vendor qualification/monitoring program
- Validated equipment, processes and test methods
- Equipment calibration and maintenance schedules using a Regulatory Asset Manager.
- Facility/Utilities maintenance, contaminations controls, safety and pest control programs
- Material review process for variances
- Robust product stability program following relevant ICH guidelines
R&D Systems strives to provide our customers with the analytical characteristics of each product so that customers may determine whether our products are appropriate for their application. Each product is provided with a lot-specific Certificate of Analysis that contains the product's specifications and test results. Quality Control testing may include, but is not limited to:
- N-terminal amino acid analysis
- SDS-PAGE purity analysis
- Molecular weight analysis via mass spectrometry
- Endotoxin assessment per USP <85> and Ph. Eur. 2.6.14 guidelines
- Bioassay analysis
- Microbial testing per USP <71> and Ph. Eur. 2.6.1 guidelines
- Host cell protein assessment
- Host cell DNA assessment
- Mycoplasma assessment
Additional testing and documentation requested by the customer can be arranged at an additional cost.
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis and St. Paul, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Product Specific Notices for Recombinant Human GDNF GMP Protein, CF
Full terms and conditions of sale can be found online in the Protein Sciences Segment T&Cs at: Terms & Conditions.
For preclinical, or clinical ex vivo use