Recombinant Human Betacellulin GMP Protein, CF GMP Best Seller
R&D Systems, part of Bio-Techne | Catalog # BT-BTC-GMP

Key Product Details
- BTC Manufactured in Bio-Techne's new GMP facility
- Lot-to-lot consistency
- Stringent guidelines for patient safety
- Scalability necessary to support successful therapeutics
- Learn more about manufacturing in our new GMP facility
- Test it in your process! Request a sample of GMP Betacellulin
Product Specifications
Source
Asp32-Gln118, with an N-terminal Met
Produced using non-animal reagents in an animal-free laboratory.
Manufactured and tested under cGMP guidelines.
Purity
Endotoxin Level
N-terminal Sequence Analysis
Predicted Molecular Mass
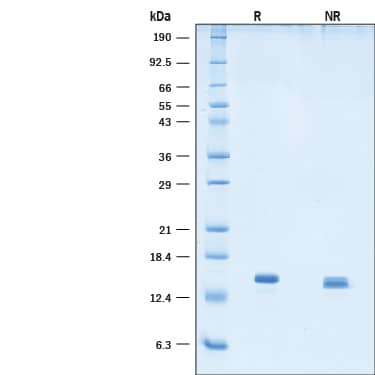
SDS-PAGE
Activity
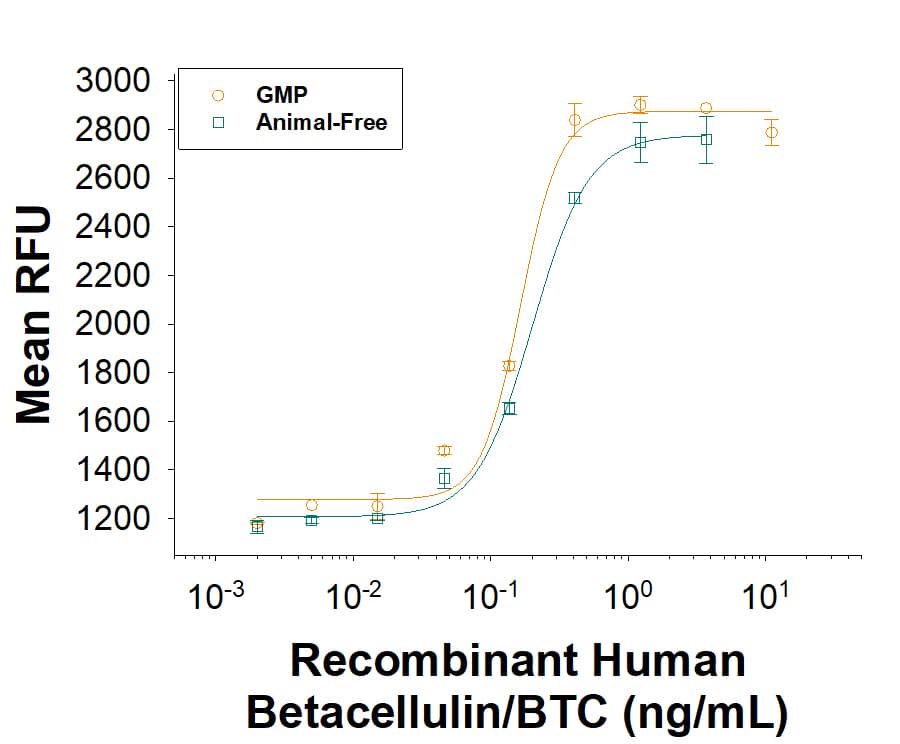
The ED50 for this effect is 0.100-1.50 ng/mL.
Host Cell Protein
Mycoplasma
Host Cell DNA
Scientific Data Images for Recombinant Human Betacellulin GMP Protein, CF
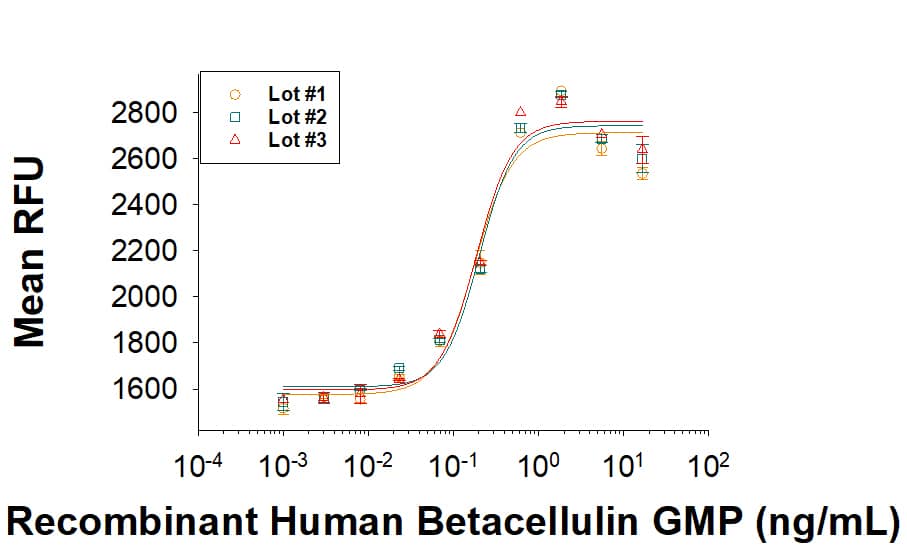
Recombinant Human Betacellulin GMP Protein Bioactivity.
GMP-grade Recombinant Human Betacellulin (Catalog # BT-BTC-GMP) as measured in a cell proliferation assay using Balb/3T3 mouse embryonic fibroblast cells. The ED50 for this effect is 0.100-1.50 ng/mL. Three independent lots were tested for activity and plotted on the same graph to show lot-to-lot consistency of GMP Betacellulin.Equivalent Bioactivity of GMP and Animal-Free grades of Recombinant Human Betacellulin/BTC.
Equivalent bioactivity of GMP (Catalog # BT-BTC-GMP) and Animal-Free (BT-BTC-AFL) grades of Recombinant Human Betacellulin/BTC as measured in a cell proliferation assay using Balb/3T3 mouse embryonic fibroblast cells (orange and green, respectively).Recombinant Human Betacellulin GMP Protein SDS-PAGE
2 ug/lane of GMP-grade Recombinant Human Betacellulin (Catalog # BT-BTC-GMP) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 13-15 kDa and 14-15 kDa, respectively.Formulation, Preparation and Storage
BT-BTC-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 100-500 μg/mL in PBS. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: Betacellulin/BTC
Betacellulin (BTC) is a new member of the EGF family of cytokines that is comprised of at least ten proteins including EGF, TGF-alpha, amphiregulin, HB-EGF, and the various heregulins. All of these cytokines are synthesized as transmembrane precursors and are characterized by the presence of one or more EGF structural units in their extracellular domain. The soluble forms of these cytokines are released by proteolytic cleavage. BTC, a heparin-binding protein, was originally isolated from the conditioned media of mouse pancreatic beta tumor cells as a 32 kDa glycoprotein composed of 80 amino acid residues. The cDNA encoding human BTC was cloned from a human breast adenocarcinoma cell line (MCF-7) cDNA library. Human and mouse cDNAs encode BTC precursor proteins of 178 and 177 amino acid residues, respectively. At the amino acid sequence level, human BTC precursor protein exhibits 79% identity with that of the mouse BTC precursor. In a mouse cell line transfected with human BTC cDNA, three forms of soluble human BTC have been detected: the glycosylated, intact BTC composed of 80 amino acid residues, a truncated molecule lacking 12 amino acid residues from the amino terminus, and a second truncated molecule lacking 30 amino acid residues from the amino terminus. The biological activities of these BTC forms were shown to be identical. BTC can bind to the EGF receptor and is a potent mitogen for Balb/c 3T3 fibroblasts, retinal pigment epithelial cells and vascular smooth muscle cells.
Additional Betacellulin/BTC Products
Product Documents for Recombinant Human Betacellulin GMP Protein, CF
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Designed, manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented and controlled manufacturing process
- Control of documentation and process changes by QA
- Personnel training programs
- Raw material inspection and vendor qualification/monitoring program
- Validated equipment, processes and test methods
- Equipment calibration and maintenance schedules using a Regulatory Asset Manager
- Facility/Utilities maintenance, contamination controls, safety and pest control programs
- Material review process for variances
- Robust product stability program following relevant ICH guidelines
- N-terminal amino acid analysis
- SDS-PAGE purity analysis
- Molecular weight analysis via mass spectrometry
- Endotoxin assessment per USP <85> and Ph. Eur. 2.6.14 guidelines
- Bioassay analysis
- Microbial testing per USP <71> and Ph. Eur. 2.6.1 guidelines
- Host cell protein assessment
- Host cell DNA assessment
- Mycoplasma assessment
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis and St. Paul, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Animal-Free Manufacturing Conditions
Our dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers.
Product Specific Notices for Recombinant Human Betacellulin GMP Protein, CF
Full terms and conditions of sale can be found online in the Protein Sciences Segment T&Cs at: Terms & Conditions.
For preclinical, or clinical ex vivo use