Recombinant Human BDNF GMP, CF GMP
R&D Systems, part of Bio-Techne | Catalog # 248-GMP

Key Product Details
Product Specifications
Source
His129-Arg247
Accession # P23560
100% sequence homology with Mouse, Rat, Canine, Equine and all other mammalian proteins examined.
Manufactured and tested under cGMP guidelines.
Purity
Endotoxin Level
N-terminal Sequence Analysis
His129-Ser-Asp-Pro-Ala-Arg-Arg-Gly-Glu-Leu
Arg134-Arg-Gly-Glu-Leu-Ser-Val-(Cys)-Asp-Ser
Predicted Molecular Mass
SDS-PAGE
Activity
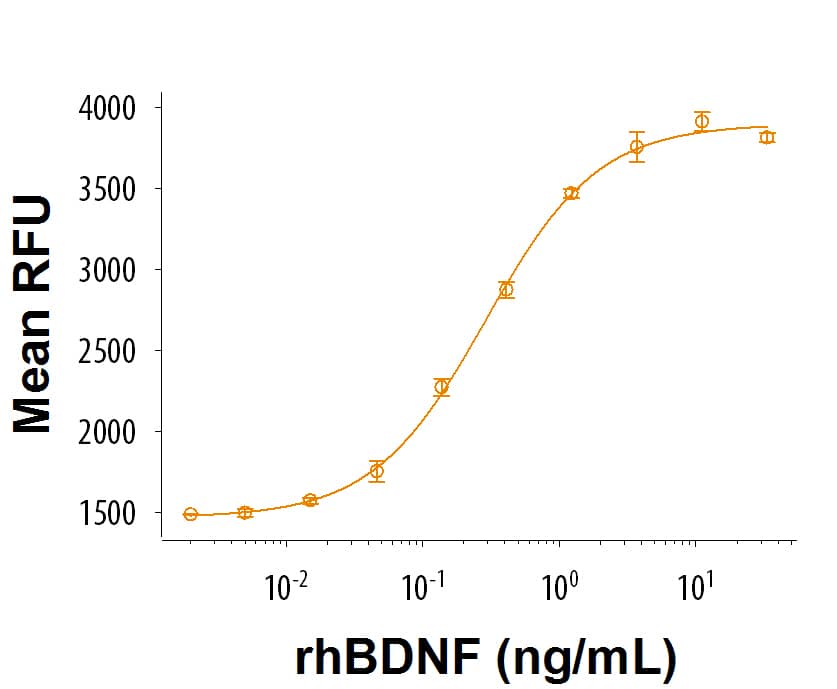
The ED50 for this effect is 0.2-2 ng/mL.
The specific activity of Recombinant Human BDNF is >3.0 x 105 units/mg, which is calibrated against the human BDNF WHO Standard (NIBSC code: 96/534).
Measured by its binding ability in a functional ELISA.
When Recombinant Human TrkB Fc Chimera (Catalog # 688-TK) is coated at 1 μg/mL, recombinant human BDNF binds with an apparent Kd <1 nM.
Mycoplasma
Scientific Data Images for Recombinant Human BDNF GMP, CF
Recombinant Human BDNF GMP Bioactivity
GMP-grade Recombinant Human BDNF (Catalog # 248-GMP) stimulates proliferation in the BaF mouse pro-B cell line transfected with TrkB. The ED50 for this effect is 0.2-2 ng/mL.Recombinant Human BDNF GMP SDS-PAGE
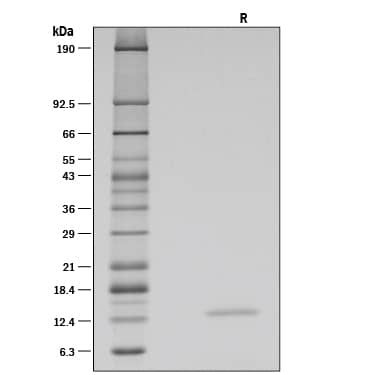
1 μg/lane of GMP-grade Recombinant Human BDNF (Catalog # 248-GMP) was resolved with SDS-PAGE under reducing (R) conditions and visualized by silver staining, showing a band at 14 kDa.Formulation, Preparation and Storage
248-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in Sodium Citrate and NaCl. |
| Reconstitution | Reconstitute at 100 μg/mL in PBS. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: BDNF
Brain-derived neurotrophic factor (BDNF) is a member of the NGF family of neurotrophic factors (also named neurotrophins) that are required for the differentiation and survival of specific neuronal subpopulations in both the central as well as the peripheral nervous system. The neurotrophin family is comprised of at least four proteins including NGF, BDNF, NT-3, and NT-4/5. These secreted cytokines are synthesized as prepropeptides that are proteolytically processed to generate the mature proteins (1, 2). All neurotrophins have six conserved cysteine residues that are involved in the formation of three disulfide bonds and all share approximately 55% sequence identity at the amino acid level. Similarly to NGF, bioactive BDNF is predicted to be a non-covalently linked homodimer.
BDNF cDNA encodes a 247 amino acid residue precursor protein with a signal peptide and a proprotein that are cleaved to yield the 119 amino acid residue mature BDNF. The amino acid sequence of mature BDNF is identical in all mammals examined. High levels of expression of BDNF have been detected in the hippocampus, cerebellum, fetal eye and placenta. In addition, low levels of BDNF expression are also found in the pituitary gland, spinal cord, heart, lung and skeletal muscle. BDNF binds with high affinity and specifically activates the TrkB tyrosine kinase receptor (3).
References
- Eide, F.F. et al. (1993) Exp. Neurol. 121:200.
- Snider, W.D. (1994) Cell 77:627.
- Barbacid, M. (1994) J. Neurobiol. 25:1386.
Long Name
Alternate Names
Gene Symbol
UniProt
Additional BDNF Products
Product Documents for Recombinant Human BDNF GMP, CF
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Designed, manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented and controlled manufacturing process
- Control of documentation and process changes by QA
- Personnel training programs
- Raw material inspection and vendor qualification/monitoring program
- Validated equipment, processes and test methods
- Equipment calibration and maintenance schedules using a Regulatory Asset Manager
- Facility/Utilities maintenance, contamination controls, safety and pest control programs
- Material review process for variances
- Robust product stability program following relevant ICH guidelines
- N-terminal amino acid analysis
- SDS-PAGE purity analysis
- Molecular weight analysis via mass spectrometry
- Endotoxin assessment per USP <85> and Ph. Eur. 2.6.14 guidelines
- Bioassay analysis
- Microbial testing per USP <71> and Ph. Eur. 2.6.1 guidelines
- Host cell protein assessment
- Host cell DNA assessment
- Mycoplasma assessment
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis and St. Paul, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Product Specific Notices for Recombinant Human BDNF GMP, CF
Full terms and conditions of sale can be found online in the Protein Sciences Segment T&Cs at: Terms & Conditions.
For preclinical, or clinical ex vivo use