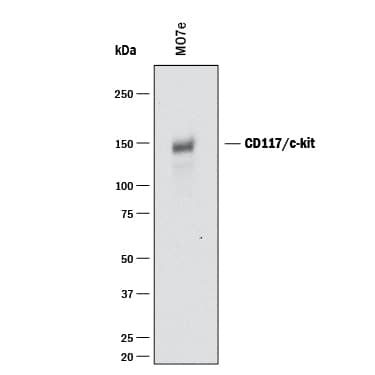
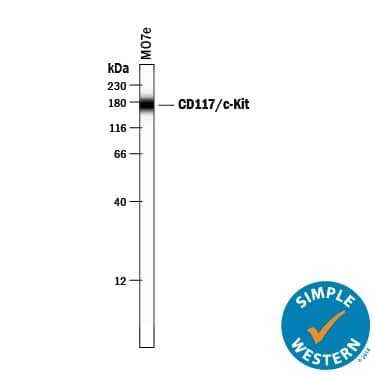
Detection of Human CD117/c-kit by Simple WesternTM.
Simple Western lane view shows lysates of MO7e human megakaryocytic leukemic cell line, loaded at 0.2 mg/mL. A specific band was detected for CD117/c-kit at approximately 172 kDa (as indicated) using 5 µg/mL of Goat Anti-Human CD117/c-kit Antigen Affinity-purified Polyclonal Antibody (Catalog # AF332) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog #
HAF109). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
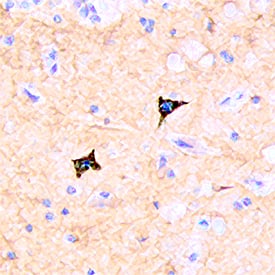
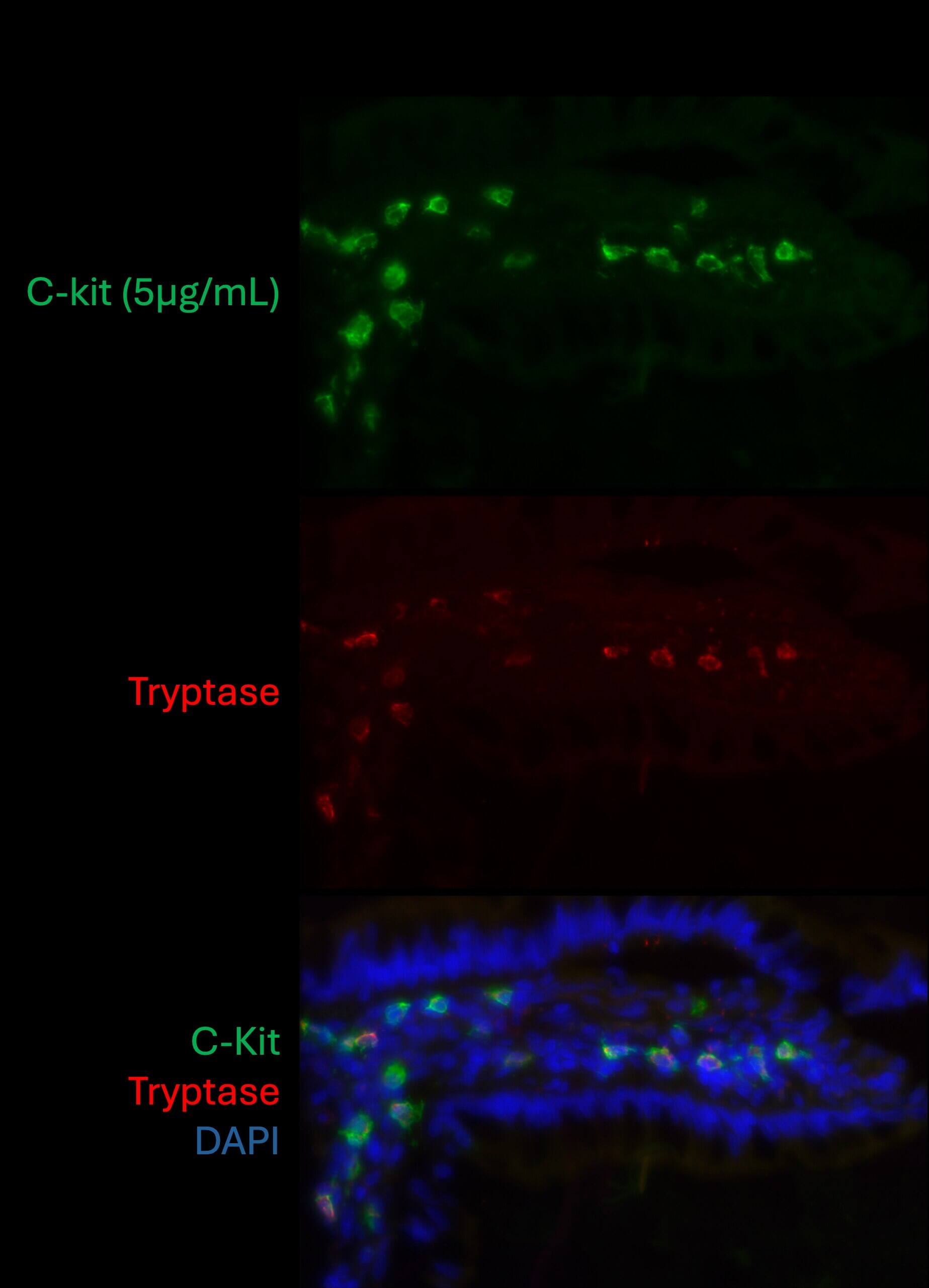
CD117/c-kit in Human Small Intestine.
Tissue fixed in 4% PFA for 1 hour, and cryoprotected in 30% sucrose for 24 hours. Tissue frozen in OCT compound and cut at 10um thickness. Blocked for 1 hour in protein block and stained at 5 ug/mL. Image from a verified customer review.
Detection of CD117/c-kit by Western Blot
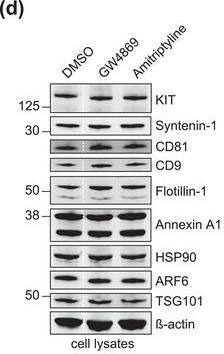
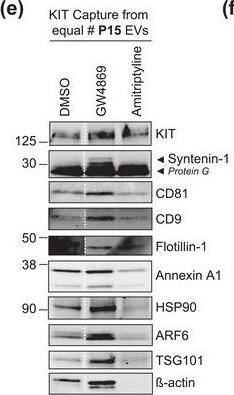
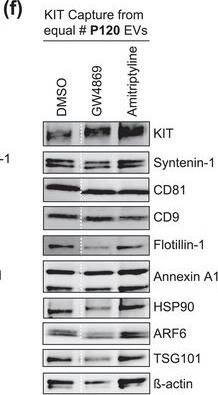
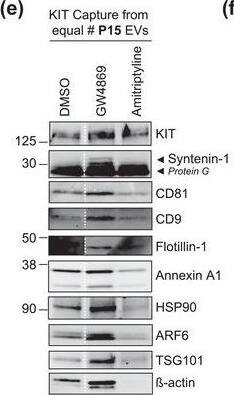
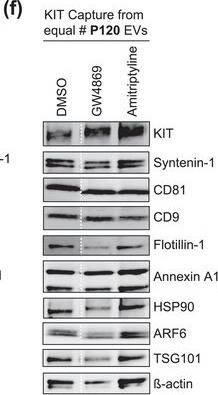
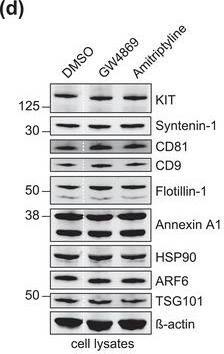
The protein loading of KIT‐EVs shifts among vesicle subtypes after cell treatment with SMase inhibitors. (A) Viability assessment of HMC‐1.1 cells at the end of the 16 h treatment with DMSO (vehicle control), 10 μM GW4869 (n‐SMase inhibitor) or 10 μM amitriptyline (a‐SMase inhibitor) to control for potential drug‐induced cell death. Data are presented as mean ± S.D. from three independent experiments, normalized to the control. (B, C) EVs released by control‐ or inhibitor‐treated HMC‐1.1 cells were pelleted as P15 (B) or P120 (C) EVs and evaluated by nanoparticle tracking. The sum of P15 (B) or P120 (C) EVs (ranging from 0 to 250 nm) released under each condition was plotted. Representative data are shown as mean ± S.D. from five repetitions. Ns, not significant; **, 0.0011; ****p < 0.0001 (two‐tailed, unpaired Student t‐test). (D) Total lysates (30 μg) of DMSO‐ or inhibitor‐treated cells were analyzed by immunoblotting with the indicated antibodies. (E,F) KIT‐EVs were captured with an antibody against N‐terminal KIT from P15 (3.4 × 108 EVs) (E) or P120 (2.6 × 109 EVs) (F) vesicles released by control, GW4869‐ or amitriptyline‐treated cells. Total KIT‐EV lysates were evaluated by Western blotting; representative blots are shown. (G,H) Signal intensities of proteins in KIT‐EVs (E,F) were quantified and normalized to the DMSO control. Up‐ or down‐regulation (indicated by arrows) of protein content in P15 and P120 KIT‐EVs in response to GW4869 (G) or amitriptyline (H) is displayed in Volcano graphs (n = 3). The q‐value is the FDR‐adjusted p‐value, and the dashed line indicates the significance threshold. In D, E and F, dashed lines imply lanes from the same blot that were not loaded contiguously Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
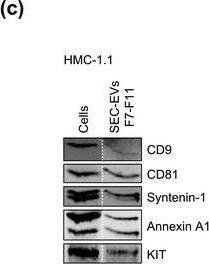
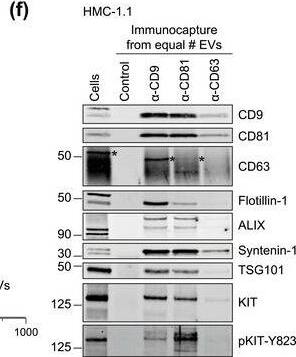
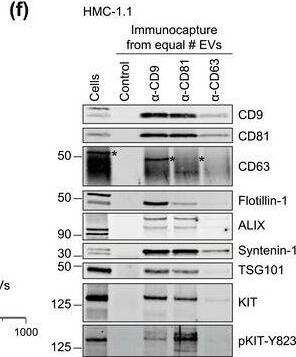
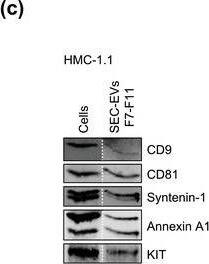
HMC‐1‐derived EVs contain KIT and canonical EV protein markers. (A, B) Detection of canonical EV marker (A) and mast cell marker proteins (B) as well as calreticulin as an exclusion marker in HMC‐1.1 and HMC‐1.2 cells and secreted EVs. 30 μg of protein of cell or EV lysates were separated by SDS‐PAGE, blotted and probed with the indicated antibodies. (C) HMC‐1.1‐derived EVs were separated from soluble protein by size exclusion chromatography (SEC; qEVoriginal70) and eluted as pooled fractions F7‐F11, followed by filter concentration. Cell (30 μg) and EV lysates (15 μg) were analyzed by immunoblotting with the indicated antibodies. The dashed line indicates the samples were run on the same blot, but not in contiguous lanes. (D) A representative graph showing the concentration and size measurement of SEC‐isolated EVs (C) by nanoparticle tracking. The average profile of five workflow repetitions is displayed. (E) Workflow of the immunocapture of EV subpopulations. EVs are bulk‐isolated from pre‐cleared cell culture media by PEG‐precipitation and incubated with antibody‐conjugated beads allowing for immunocapture of specific EV subpopulations. (F) Representative Western blot of CD9‐, CD81‐ and CD63‐immunocaptured EVs. Each bead capture was performed from an equal number of HMC‐1.1‐derived EVs (1.9 × 1010 EVs). Unconjugated beads served as a negative control. The presence of canonical EV protein markers as well as KIT in the EV subpopulations were determined by immunoblotting. *, signal from previous probing with a flotillin‐1 antibody Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
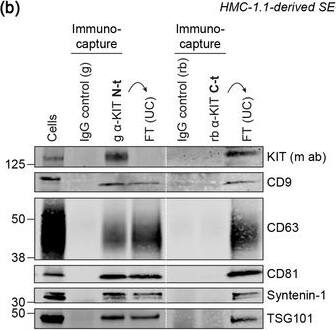
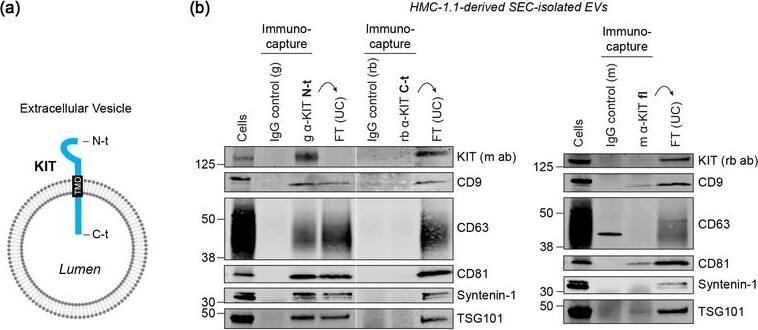
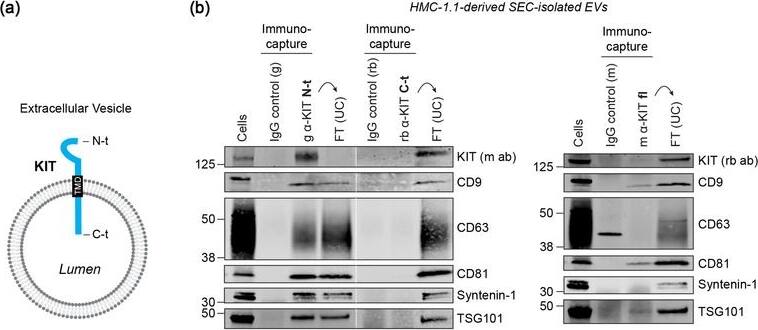
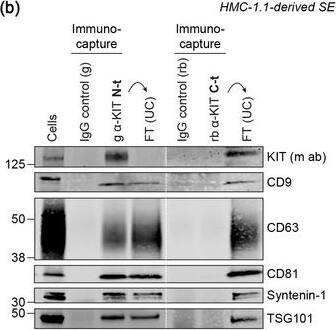
KIT‐containing EVs can be isolated by immunocapture. (B) Representative immunoblots showing the canonical EV marker content of KIT‐EVs captured from HMC‐1.1‐derived EVs that isolated by size exclusion chromatography (SEC). Three different antibodies directed against N‐t, C‐t (left panel) or full‐length (fl) KIT protein (right panel) used for immunocapturing of KIT‐containing EVs. Each pulldown was performed from vesicles secreted by 20 × 106 & collected in SEC fractions seven to nine. Unbound EVs in the flow‐through (FT) pelleted by ultracentrifugation (UC) to demonstrate the capture efficiency. Beads conjugated to isotype control antibodies served as negative controls. Cell lysates (30 μg) included. g, goat; rb, rabbit; m, mouse. Dashed lines imply lanes from the same blot that not loaded contiguously. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
HMC‐1‐derived EVs contain KIT and canonical EV protein markers. (A, B) Detection of canonical EV marker (A) and mast cell marker proteins (B) as well as calreticulin as an exclusion marker in HMC‐1.1 and HMC‐1.2 cells and secreted EVs. 30 μg of protein of cell or EV lysates were separated by SDS‐PAGE, blotted and probed with the indicated antibodies. (C) HMC‐1.1‐derived EVs were separated from soluble protein by size exclusion chromatography (SEC; qEVoriginal70) and eluted as pooled fractions F7‐F11, followed by filter concentration. Cell (30 μg) and EV lysates (15 μg) were analyzed by immunoblotting with the indicated antibodies. The dashed line indicates the samples were run on the same blot, but not in contiguous lanes. (D) A representative graph showing the concentration and size measurement of SEC‐isolated EVs (C) by nanoparticle tracking. The average profile of five workflow repetitions is displayed. (E) Workflow of the immunocapture of EV subpopulations. EVs are bulk‐isolated from pre‐cleared cell culture media by PEG‐precipitation and incubated with antibody‐conjugated beads allowing for immunocapture of specific EV subpopulations. (F) Representative Western blot of CD9‐, CD81‐ and CD63‐immunocaptured EVs. Each bead capture was performed from an equal number of HMC‐1.1‐derived EVs (1.9 × 1010 EVs). Unconjugated beads served as a negative control. The presence of canonical EV protein markers as well as KIT in the EV subpopulations were determined by immunoblotting. *, signal from previous probing with a flotillin‐1 antibody Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
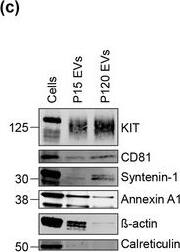
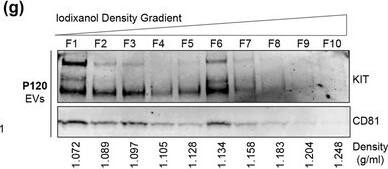
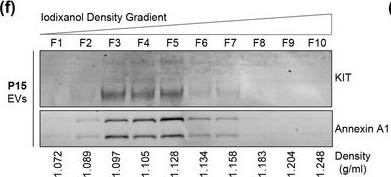
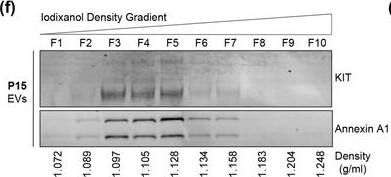
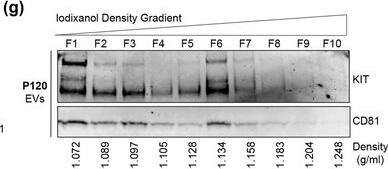
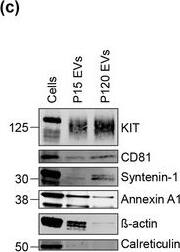
KIT‐EVs are released as heterogeneous subpopulations with different physical properties. (A) Cell culture supernatant was subjected to differential ultracentrifugation to obtain P15 and P120 EV pellets, as illustrated. Where indicated, P15 and P120 EVs were further separated by iodixanol density gradients. Vesicles from each density fraction were pelleted and analyzed. (B) P15 and P120 EVs obtained from HMC‐1.1 cells were analyzed by nanoparticle tracking. Representative concentration and size profiles averaged from five workflow repetitions are depicted. The averaged percentage (%) of different size ranges within the P15 or P120 population is shown on top (bar chart). (C) Representative Western blot of HMC‐1.1 cell, P15 and P120 EV lysates (30 μg) probed with the indicated antibodies. (D, E) HMC‐1.1‐derived P15 (D) or P120 (E) EV pellets were separated by density gradients and the vesicles from each fraction (F1‐F10) were analyzed by nanoparticle tracking for concentration (particles/ml), shown as box plots, and size (represented as average mode, nm), shown as a line graph. Data in the box plots represent the median with the whiskers indicating minimum and maximum values; data in the line graph are represented as mean ± S.D. from five repetitions. Representative results are shown. (F, G) Representative immunoblots showing the presence of KIT and annexin A1 in P15 EV fractions (F) and KIT and CD81 in P120 EV fractions (G). The density (g/ml) of each fraction is indicated below Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
The protein loading of KIT‐EVs shifts among vesicle subtypes after cell treatment with SMase inhibitors. (A) Viability assessment of HMC‐1.1 cells at the end of the 16 h treatment with DMSO (vehicle control), 10 μM GW4869 (n‐SMase inhibitor) or 10 μM amitriptyline (a‐SMase inhibitor) to control for potential drug‐induced cell death. Data are presented as mean ± S.D. from three independent experiments, normalized to the control. (B, C) EVs released by control‐ or inhibitor‐treated HMC‐1.1 cells were pelleted as P15 (B) or P120 (C) EVs and evaluated by nanoparticle tracking. The sum of P15 (B) or P120 (C) EVs (ranging from 0 to 250 nm) released under each condition was plotted. Representative data are shown as mean ± S.D. from five repetitions. Ns, not significant; **, 0.0011; ****p < 0.0001 (two‐tailed, unpaired Student t‐test). (D) Total lysates (30 μg) of DMSO‐ or inhibitor‐treated cells were analyzed by immunoblotting with the indicated antibodies. (E,F) KIT‐EVs were captured with an antibody against N‐terminal KIT from P15 (3.4 × 108 EVs) (E) or P120 (2.6 × 109 EVs) (F) vesicles released by control, GW4869‐ or amitriptyline‐treated cells. Total KIT‐EV lysates were evaluated by Western blotting; representative blots are shown. (G,H) Signal intensities of proteins in KIT‐EVs (E,F) were quantified and normalized to the DMSO control. Up‐ or down‐regulation (indicated by arrows) of protein content in P15 and P120 KIT‐EVs in response to GW4869 (G) or amitriptyline (H) is displayed in Volcano graphs (n = 3). The q‐value is the FDR‐adjusted p‐value, and the dashed line indicates the significance threshold. In D, E and F, dashed lines imply lanes from the same blot that were not loaded contiguously Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
KIT‐EVs are released as heterogeneous subpopulations with different physical properties. (A) Cell culture supernatant was subjected to differential ultracentrifugation to obtain P15 and P120 EV pellets, as illustrated. Where indicated, P15 and P120 EVs were further separated by iodixanol density gradients. Vesicles from each density fraction were pelleted and analyzed. (B) P15 and P120 EVs obtained from HMC‐1.1 cells were analyzed by nanoparticle tracking. Representative concentration and size profiles averaged from five workflow repetitions are depicted. The averaged percentage (%) of different size ranges within the P15 or P120 population is shown on top (bar chart). (C) Representative Western blot of HMC‐1.1 cell, P15 and P120 EV lysates (30 μg) probed with the indicated antibodies. (D, E) HMC‐1.1‐derived P15 (D) or P120 (E) EV pellets were separated by density gradients and the vesicles from each fraction (F1‐F10) were analyzed by nanoparticle tracking for concentration (particles/ml), shown as box plots, and size (represented as average mode, nm), shown as a line graph. Data in the box plots represent the median with the whiskers indicating minimum and maximum values; data in the line graph are represented as mean ± S.D. from five repetitions. Representative results are shown. (F, G) Representative immunoblots showing the presence of KIT and annexin A1 in P15 EV fractions (F) and KIT and CD81 in P120 EV fractions (G). The density (g/ml) of each fraction is indicated below Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
KIT‐EVs are released as heterogeneous subpopulations with different physical properties. (A) Cell culture supernatant was subjected to differential ultracentrifugation to obtain P15 and P120 EV pellets, as illustrated. Where indicated, P15 and P120 EVs were further separated by iodixanol density gradients. Vesicles from each density fraction were pelleted and analyzed. (B) P15 and P120 EVs obtained from HMC‐1.1 cells were analyzed by nanoparticle tracking. Representative concentration and size profiles averaged from five workflow repetitions are depicted. The averaged percentage (%) of different size ranges within the P15 or P120 population is shown on top (bar chart). (C) Representative Western blot of HMC‐1.1 cell, P15 and P120 EV lysates (30 μg) probed with the indicated antibodies. (D, E) HMC‐1.1‐derived P15 (D) or P120 (E) EV pellets were separated by density gradients and the vesicles from each fraction (F1‐F10) were analyzed by nanoparticle tracking for concentration (particles/ml), shown as box plots, and size (represented as average mode, nm), shown as a line graph. Data in the box plots represent the median with the whiskers indicating minimum and maximum values; data in the line graph are represented as mean ± S.D. from five repetitions. Representative results are shown. (F, G) Representative immunoblots showing the presence of KIT and annexin A1 in P15 EV fractions (F) and KIT and CD81 in P120 EV fractions (G). The density (g/ml) of each fraction is indicated below Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
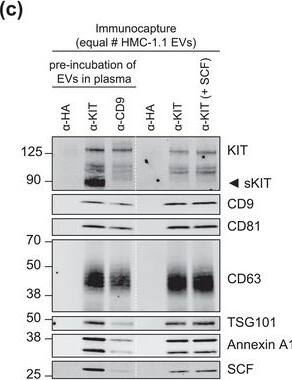
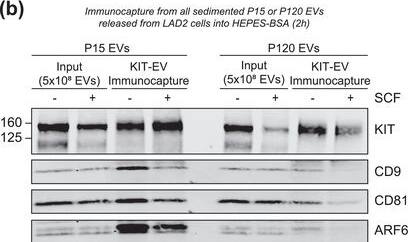
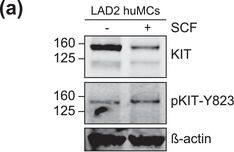
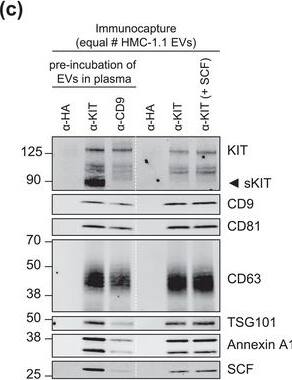
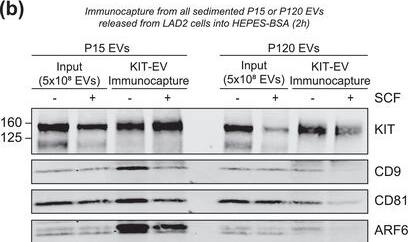
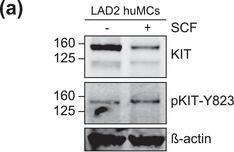
KIT‐EV immunocapture of LAD2 huMC EVs and KIT‐EV recovery from spiked plasma. (A) Cytokine‐starved LAD2 huMCs were treated or not with stem cell factor (SCF; 100 ng/ml) for 2 h in HEPES‐BSA buffer. Cell lysates (30 μg) were analyzed by immunoblotting with the indicated antibodies. (B) EVs released into the supernatant by 18 × 106 LAD2 cells treated or not with SCF for 2 h were sedimented by differential ultracentrifugation into P15 and P120 EVs. KIT‐EV immunocapture was performed (KIT antibody AF332) from all sedimented LAD2 P15 and P120 EVs. The total bead elution was loaded and analyzed by Western blotting with the indicated antibodies. Input EV lysates (5 × 108) were included. Representative blots are shown. (C) HMC‐1.1 cell line‐derived EVs (1.2 × 1010 EVs) were incubated or not in human normal plasma and captured with a KIT antibody (AF332) or by CD9 antibody‐conjugated beads. A goat HA antibody served as a negative isotype control. Representative blots are shown. sKIT, soluble KIT that is present in plasma. Dashed lines imply lanes from the same blot that were not loaded contiguously Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
HMC‐1‐derived EVs contain KIT and canonical EV protein markers. (A, B) Detection of canonical EV marker (A) and mast cell marker proteins (B) as well as calreticulin as an exclusion marker in HMC‐1.1 and HMC‐1.2 cells and secreted EVs. 30 μg of protein of cell or EV lysates were separated by SDS‐PAGE, blotted and probed with the indicated antibodies. (C) HMC‐1.1‐derived EVs were separated from soluble protein by size exclusion chromatography (SEC; qEVoriginal70) and eluted as pooled fractions F7‐F11, followed by filter concentration. Cell (30 μg) and EV lysates (15 μg) were analyzed by immunoblotting with the indicated antibodies. The dashed line indicates the samples were run on the same blot, but not in contiguous lanes. (D) A representative graph showing the concentration and size measurement of SEC‐isolated EVs (C) by nanoparticle tracking. The average profile of five workflow repetitions is displayed. (E) Workflow of the immunocapture of EV subpopulations. EVs are bulk‐isolated from pre‐cleared cell culture media by PEG‐precipitation and incubated with antibody‐conjugated beads allowing for immunocapture of specific EV subpopulations. (F) Representative Western blot of CD9‐, CD81‐ and CD63‐immunocaptured EVs. Each bead capture was performed from an equal number of HMC‐1.1‐derived EVs (1.9 × 1010 EVs). Unconjugated beads served as a negative control. The presence of canonical EV protein markers as well as KIT in the EV subpopulations were determined by immunoblotting. *, signal from previous probing with a flotillin‐1 antibody Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
HMC‐1‐derived EVs contain KIT and canonical EV protein markers. (A, B) Detection of canonical EV marker (A) and mast cell marker proteins (B) as well as calreticulin as an exclusion marker in HMC‐1.1 and HMC‐1.2 cells and secreted EVs. 30 μg of protein of cell or EV lysates were separated by SDS‐PAGE, blotted and probed with the indicated antibodies. (C) HMC‐1.1‐derived EVs were separated from soluble protein by size exclusion chromatography (SEC; qEVoriginal70) and eluted as pooled fractions F7‐F11, followed by filter concentration. Cell (30 μg) and EV lysates (15 μg) were analyzed by immunoblotting with the indicated antibodies. The dashed line indicates the samples were run on the same blot, but not in contiguous lanes. (D) A representative graph showing the concentration and size measurement of SEC‐isolated EVs (C) by nanoparticle tracking. The average profile of five workflow repetitions is displayed. (E) Workflow of the immunocapture of EV subpopulations. EVs are bulk‐isolated from pre‐cleared cell culture media by PEG‐precipitation and incubated with antibody‐conjugated beads allowing for immunocapture of specific EV subpopulations. (F) Representative Western blot of CD9‐, CD81‐ and CD63‐immunocaptured EVs. Each bead capture was performed from an equal number of HMC‐1.1‐derived EVs (1.9 × 1010 EVs). Unconjugated beads served as a negative control. The presence of canonical EV protein markers as well as KIT in the EV subpopulations were determined by immunoblotting. *, signal from previous probing with a flotillin‐1 antibody Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
KIT‐containing EVs can be isolated by immunocapture. (A) Illustration of the predicted topology of the receptor KIT in an EV. KIT is embedded in the EV membrane by its transmembrane domain (TMD) with the N‐terminal (N‐t) ligand‐binding domain exposed to the extravesicular environment, while the C‐terminal (C‐t) protein domain is directed into the EV lumen. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
KIT‐EV immunocapture of LAD2 huMC EVs and KIT‐EV recovery from spiked plasma. (A) Cytokine‐starved LAD2 huMCs were treated or not with stem cell factor (SCF; 100 ng/ml) for 2 h in HEPES‐BSA buffer. Cell lysates (30 μg) were analyzed by immunoblotting with the indicated antibodies. (B) EVs released into the supernatant by 18 × 106 LAD2 cells treated or not with SCF for 2 h were sedimented by differential ultracentrifugation into P15 and P120 EVs. KIT‐EV immunocapture was performed (KIT antibody AF332) from all sedimented LAD2 P15 and P120 EVs. The total bead elution was loaded and analyzed by Western blotting with the indicated antibodies. Input EV lysates (5 × 108) were included. Representative blots are shown. (C) HMC‐1.1 cell line‐derived EVs (1.2 × 1010 EVs) were incubated or not in human normal plasma and captured with a KIT antibody (AF332) or by CD9 antibody‐conjugated beads. A goat HA antibody served as a negative isotype control. Representative blots are shown. sKIT, soluble KIT that is present in plasma. Dashed lines imply lanes from the same blot that were not loaded contiguously Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
KIT‐containing EVs can be isolated by immunocapture. (A) Illustration of the predicted topology of the receptor KIT in an EV. KIT is embedded in the EV membrane by its transmembrane domain (TMD) with the N‐terminal (N‐t) ligand‐binding domain exposed to the extravesicular environment, while the C‐terminal (C‐t) protein domain is directed into the EV lumen. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
KIT‐EVs are released as heterogeneous subpopulations with different physical properties. (A) Cell culture supernatant was subjected to differential ultracentrifugation to obtain P15 and P120 EV pellets, as illustrated. Where indicated, P15 and P120 EVs were further separated by iodixanol density gradients. Vesicles from each density fraction were pelleted and analyzed. (B) P15 and P120 EVs obtained from HMC‐1.1 cells were analyzed by nanoparticle tracking. Representative concentration and size profiles averaged from five workflow repetitions are depicted. The averaged percentage (%) of different size ranges within the P15 or P120 population is shown on top (bar chart). (C) Representative Western blot of HMC‐1.1 cell, P15 and P120 EV lysates (30 μg) probed with the indicated antibodies. (D, E) HMC‐1.1‐derived P15 (D) or P120 (E) EV pellets were separated by density gradients and the vesicles from each fraction (F1‐F10) were analyzed by nanoparticle tracking for concentration (particles/ml), shown as box plots, and size (represented as average mode, nm), shown as a line graph. Data in the box plots represent the median with the whiskers indicating minimum and maximum values; data in the line graph are represented as mean ± S.D. from five repetitions. Representative results are shown. (F, G) Representative immunoblots showing the presence of KIT and annexin A1 in P15 EV fractions (F) and KIT and CD81 in P120 EV fractions (G). The density (g/ml) of each fraction is indicated below Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
KIT‐EV immunocapture of LAD2 huMC EVs and KIT‐EV recovery from spiked plasma. (A) Cytokine‐starved LAD2 huMCs were treated or not with stem cell factor (SCF; 100 ng/ml) for 2 h in HEPES‐BSA buffer. Cell lysates (30 μg) were analyzed by immunoblotting with the indicated antibodies. (B) EVs released into the supernatant by 18 × 106 LAD2 cells treated or not with SCF for 2 h were sedimented by differential ultracentrifugation into P15 and P120 EVs. KIT‐EV immunocapture was performed (KIT antibody AF332) from all sedimented LAD2 P15 and P120 EVs. The total bead elution was loaded and analyzed by Western blotting with the indicated antibodies. Input EV lysates (5 × 108) were included. Representative blots are shown. (C) HMC‐1.1 cell line‐derived EVs (1.2 × 1010 EVs) were incubated or not in human normal plasma and captured with a KIT antibody (AF332) or by CD9 antibody‐conjugated beads. A goat HA antibody served as a negative isotype control. Representative blots are shown. sKIT, soluble KIT that is present in plasma. Dashed lines imply lanes from the same blot that were not loaded contiguously Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
KIT‐EV immunocapture of LAD2 huMC EVs and KIT‐EV recovery from spiked plasma. (A) Cytokine‐starved LAD2 huMCs were treated or not with stem cell factor (SCF; 100 ng/ml) for 2 h in HEPES‐BSA buffer. Cell lysates (30 μg) were analyzed by immunoblotting with the indicated antibodies. (B) EVs released into the supernatant by 18 × 106 LAD2 cells treated or not with SCF for 2 h were sedimented by differential ultracentrifugation into P15 and P120 EVs. KIT‐EV immunocapture was performed (KIT antibody AF332) from all sedimented LAD2 P15 and P120 EVs. The total bead elution was loaded and analyzed by Western blotting with the indicated antibodies. Input EV lysates (5 × 108) were included. Representative blots are shown. (C) HMC‐1.1 cell line‐derived EVs (1.2 × 1010 EVs) were incubated or not in human normal plasma and captured with a KIT antibody (AF332) or by CD9 antibody‐conjugated beads. A goat HA antibody served as a negative isotype control. Representative blots are shown. sKIT, soluble KIT that is present in plasma. Dashed lines imply lanes from the same blot that were not loaded contiguously Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
The protein loading of KIT‐EVs shifts among vesicle subtypes after cell treatment with SMase inhibitors. (A) Viability assessment of HMC‐1.1 cells at the end of the 16 h treatment with DMSO (vehicle control), 10 μM GW4869 (n‐SMase inhibitor) or 10 μM amitriptyline (a‐SMase inhibitor) to control for potential drug‐induced cell death. Data are presented as mean ± S.D. from three independent experiments, normalized to the control. (B, C) EVs released by control‐ or inhibitor‐treated HMC‐1.1 cells were pelleted as P15 (B) or P120 (C) EVs and evaluated by nanoparticle tracking. The sum of P15 (B) or P120 (C) EVs (ranging from 0 to 250 nm) released under each condition was plotted. Representative data are shown as mean ± S.D. from five repetitions. Ns, not significant; **, 0.0011; ****p < 0.0001 (two‐tailed, unpaired Student t‐test). (D) Total lysates (30 μg) of DMSO‐ or inhibitor‐treated cells were analyzed by immunoblotting with the indicated antibodies. (E,F) KIT‐EVs were captured with an antibody against N‐terminal KIT from P15 (3.4 × 108 EVs) (E) or P120 (2.6 × 109 EVs) (F) vesicles released by control, GW4869‐ or amitriptyline‐treated cells. Total KIT‐EV lysates were evaluated by Western blotting; representative blots are shown. (G,H) Signal intensities of proteins in KIT‐EVs (E,F) were quantified and normalized to the DMSO control. Up‐ or down‐regulation (indicated by arrows) of protein content in P15 and P120 KIT‐EVs in response to GW4869 (G) or amitriptyline (H) is displayed in Volcano graphs (n = 3). The q‐value is the FDR‐adjusted p‐value, and the dashed line indicates the significance threshold. In D, E and F, dashed lines imply lanes from the same blot that were not loaded contiguously Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
KIT‐EVs are released as heterogeneous subpopulations with different physical properties. (A) Cell culture supernatant was subjected to differential ultracentrifugation to obtain P15 and P120 EV pellets, as illustrated. Where indicated, P15 and P120 EVs were further separated by iodixanol density gradients. Vesicles from each density fraction were pelleted and analyzed. (B) P15 and P120 EVs obtained from HMC‐1.1 cells were analyzed by nanoparticle tracking. Representative concentration and size profiles averaged from five workflow repetitions are depicted. The averaged percentage (%) of different size ranges within the P15 or P120 population is shown on top (bar chart). (C) Representative Western blot of HMC‐1.1 cell, P15 and P120 EV lysates (30 μg) probed with the indicated antibodies. (D, E) HMC‐1.1‐derived P15 (D) or P120 (E) EV pellets were separated by density gradients and the vesicles from each fraction (F1‐F10) were analyzed by nanoparticle tracking for concentration (particles/ml), shown as box plots, and size (represented as average mode, nm), shown as a line graph. Data in the box plots represent the median with the whiskers indicating minimum and maximum values; data in the line graph are represented as mean ± S.D. from five repetitions. Representative results are shown. (F, G) Representative immunoblots showing the presence of KIT and annexin A1 in P15 EV fractions (F) and KIT and CD81 in P120 EV fractions (G). The density (g/ml) of each fraction is indicated below Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
The protein loading of KIT‐EVs shifts among vesicle subtypes after cell treatment with SMase inhibitors. (A) Viability assessment of HMC‐1.1 cells at the end of the 16 h treatment with DMSO (vehicle control), 10 μM GW4869 (n‐SMase inhibitor) or 10 μM amitriptyline (a‐SMase inhibitor) to control for potential drug‐induced cell death. Data are presented as mean ± S.D. from three independent experiments, normalized to the control. (B, C) EVs released by control‐ or inhibitor‐treated HMC‐1.1 cells were pelleted as P15 (B) or P120 (C) EVs and evaluated by nanoparticle tracking. The sum of P15 (B) or P120 (C) EVs (ranging from 0 to 250 nm) released under each condition was plotted. Representative data are shown as mean ± S.D. from five repetitions. Ns, not significant; **, 0.0011; ****p < 0.0001 (two‐tailed, unpaired Student t‐test). (D) Total lysates (30 μg) of DMSO‐ or inhibitor‐treated cells were analyzed by immunoblotting with the indicated antibodies. (E,F) KIT‐EVs were captured with an antibody against N‐terminal KIT from P15 (3.4 × 108 EVs) (E) or P120 (2.6 × 109 EVs) (F) vesicles released by control, GW4869‐ or amitriptyline‐treated cells. Total KIT‐EV lysates were evaluated by Western blotting; representative blots are shown. (G,H) Signal intensities of proteins in KIT‐EVs (E,F) were quantified and normalized to the DMSO control. Up‐ or down‐regulation (indicated by arrows) of protein content in P15 and P120 KIT‐EVs in response to GW4869 (G) or amitriptyline (H) is displayed in Volcano graphs (n = 3). The q‐value is the FDR‐adjusted p‐value, and the dashed line indicates the significance threshold. In D, E and F, dashed lines imply lanes from the same blot that were not loaded contiguously Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
KIT‐containing EVs can be isolated by immunocapture. (B) Representative immunoblots showing the canonical EV marker content of KIT‐EVs captured from HMC‐1.1‐derived EVs that isolated by size exclusion chromatography (SEC). Three different antibodies directed against N‐t, C‐t (left panel) or full‐length (fl) KIT protein (right panel) used for immunocapturing of KIT‐containing EVs. Each pulldown was performed from vesicles secreted by 20 × 106 & collected in SEC fractions seven to nine. Unbound EVs in the flow‐through (FT) pelleted by ultracentrifugation (UC) to demonstrate the capture efficiency. Beads conjugated to isotype control antibodies served as negative controls. Cell lysates (30 μg) included. g, goat; rb, rabbit; m, mouse. Dashed lines imply lanes from the same blot that not loaded contiguously. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
The protein loading of KIT‐EVs shifts among vesicle subtypes after cell treatment with SMase inhibitors. (A) Viability assessment of HMC‐1.1 cells at the end of the 16 h treatment with DMSO (vehicle control), 10 μM GW4869 (n‐SMase inhibitor) or 10 μM amitriptyline (a‐SMase inhibitor) to control for potential drug‐induced cell death. Data are presented as mean ± S.D. from three independent experiments, normalized to the control. (B, C) EVs released by control‐ or inhibitor‐treated HMC‐1.1 cells were pelleted as P15 (B) or P120 (C) EVs and evaluated by nanoparticle tracking. The sum of P15 (B) or P120 (C) EVs (ranging from 0 to 250 nm) released under each condition was plotted. Representative data are shown as mean ± S.D. from five repetitions. Ns, not significant; **, 0.0011; ****p < 0.0001 (two‐tailed, unpaired Student t‐test). (D) Total lysates (30 μg) of DMSO‐ or inhibitor‐treated cells were analyzed by immunoblotting with the indicated antibodies. (E,F) KIT‐EVs were captured with an antibody against N‐terminal KIT from P15 (3.4 × 108 EVs) (E) or P120 (2.6 × 109 EVs) (F) vesicles released by control, GW4869‐ or amitriptyline‐treated cells. Total KIT‐EV lysates were evaluated by Western blotting; representative blots are shown. (G,H) Signal intensities of proteins in KIT‐EVs (E,F) were quantified and normalized to the DMSO control. Up‐ or down‐regulation (indicated by arrows) of protein content in P15 and P120 KIT‐EVs in response to GW4869 (G) or amitriptyline (H) is displayed in Volcano graphs (n = 3). The q‐value is the FDR‐adjusted p‐value, and the dashed line indicates the significance threshold. In D, E and F, dashed lines imply lanes from the same blot that were not loaded contiguously Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
KIT‐EV immunocapture of LAD2 huMC EVs and KIT‐EV recovery from spiked plasma. (A) Cytokine‐starved LAD2 huMCs were treated or not with stem cell factor (SCF; 100 ng/ml) for 2 h in HEPES‐BSA buffer. Cell lysates (30 μg) were analyzed by immunoblotting with the indicated antibodies. (B) EVs released into the supernatant by 18 × 106 LAD2 cells treated or not with SCF for 2 h were sedimented by differential ultracentrifugation into P15 and P120 EVs. KIT‐EV immunocapture was performed (KIT antibody AF332) from all sedimented LAD2 P15 and P120 EVs. The total bead elution was loaded and analyzed by Western blotting with the indicated antibodies. Input EV lysates (5 × 108) were included. Representative blots are shown. (C) HMC‐1.1 cell line‐derived EVs (1.2 × 1010 EVs) were incubated or not in human normal plasma and captured with a KIT antibody (AF332) or by CD9 antibody‐conjugated beads. A goat HA antibody served as a negative isotype control. Representative blots are shown. sKIT, soluble KIT that is present in plasma. Dashed lines imply lanes from the same blot that were not loaded contiguously Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
The protein loading of KIT‐EVs shifts among vesicle subtypes after cell treatment with SMase inhibitors. (A) Viability assessment of HMC‐1.1 cells at the end of the 16 h treatment with DMSO (vehicle control), 10 μM GW4869 (n‐SMase inhibitor) or 10 μM amitriptyline (a‐SMase inhibitor) to control for potential drug‐induced cell death. Data are presented as mean ± S.D. from three independent experiments, normalized to the control. (B, C) EVs released by control‐ or inhibitor‐treated HMC‐1.1 cells were pelleted as P15 (B) or P120 (C) EVs and evaluated by nanoparticle tracking. The sum of P15 (B) or P120 (C) EVs (ranging from 0 to 250 nm) released under each condition was plotted. Representative data are shown as mean ± S.D. from five repetitions. Ns, not significant; **, 0.0011; ****p < 0.0001 (two‐tailed, unpaired Student t‐test). (D) Total lysates (30 μg) of DMSO‐ or inhibitor‐treated cells were analyzed by immunoblotting with the indicated antibodies. (E,F) KIT‐EVs were captured with an antibody against N‐terminal KIT from P15 (3.4 × 108 EVs) (E) or P120 (2.6 × 109 EVs) (F) vesicles released by control, GW4869‐ or amitriptyline‐treated cells. Total KIT‐EV lysates were evaluated by Western blotting; representative blots are shown. (G,H) Signal intensities of proteins in KIT‐EVs (E,F) were quantified and normalized to the DMSO control. Up‐ or down‐regulation (indicated by arrows) of protein content in P15 and P120 KIT‐EVs in response to GW4869 (G) or amitriptyline (H) is displayed in Volcano graphs (n = 3). The q‐value is the FDR‐adjusted p‐value, and the dashed line indicates the significance threshold. In D, E and F, dashed lines imply lanes from the same blot that were not loaded contiguously Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
KIT‐EV immunocapture of LAD2 huMC EVs and KIT‐EV recovery from spiked plasma. (A) Cytokine‐starved LAD2 huMCs were treated or not with stem cell factor (SCF; 100 ng/ml) for 2 h in HEPES‐BSA buffer. Cell lysates (30 μg) were analyzed by immunoblotting with the indicated antibodies. (B) EVs released into the supernatant by 18 × 106 LAD2 cells treated or not with SCF for 2 h were sedimented by differential ultracentrifugation into P15 and P120 EVs. KIT‐EV immunocapture was performed (KIT antibody AF332) from all sedimented LAD2 P15 and P120 EVs. The total bead elution was loaded and analyzed by Western blotting with the indicated antibodies. Input EV lysates (5 × 108) were included. Representative blots are shown. (C) HMC‐1.1 cell line‐derived EVs (1.2 × 1010 EVs) were incubated or not in human normal plasma and captured with a KIT antibody (AF332) or by CD9 antibody‐conjugated beads. A goat HA antibody served as a negative isotype control. Representative blots are shown. sKIT, soluble KIT that is present in plasma. Dashed lines imply lanes from the same blot that were not loaded contiguously Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.
Detection of CD117/c-kit by Western Blot
KIT‐EVs are released as heterogeneous subpopulations with different physical properties. (A) Cell culture supernatant was subjected to differential ultracentrifugation to obtain P15 and P120 EV pellets, as illustrated. Where indicated, P15 and P120 EVs were further separated by iodixanol density gradients. Vesicles from each density fraction were pelleted and analyzed. (B) P15 and P120 EVs obtained from HMC‐1.1 cells were analyzed by nanoparticle tracking. Representative concentration and size profiles averaged from five workflow repetitions are depicted. The averaged percentage (%) of different size ranges within the P15 or P120 population is shown on top (bar chart). (C) Representative Western blot of HMC‐1.1 cell, P15 and P120 EV lysates (30 μg) probed with the indicated antibodies. (D, E) HMC‐1.1‐derived P15 (D) or P120 (E) EV pellets were separated by density gradients and the vesicles from each fraction (F1‐F10) were analyzed by nanoparticle tracking for concentration (particles/ml), shown as box plots, and size (represented as average mode, nm), shown as a line graph. Data in the box plots represent the median with the whiskers indicating minimum and maximum values; data in the line graph are represented as mean ± S.D. from five repetitions. Representative results are shown. (F, G) Representative immunoblots showing the presence of KIT and annexin A1 in P15 EV fractions (F) and KIT and CD81 in P120 EV fractions (G). The density (g/ml) of each fraction is indicated below Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36239715), licensed under a CC-BY license. Not internally tested by R&D Systems.