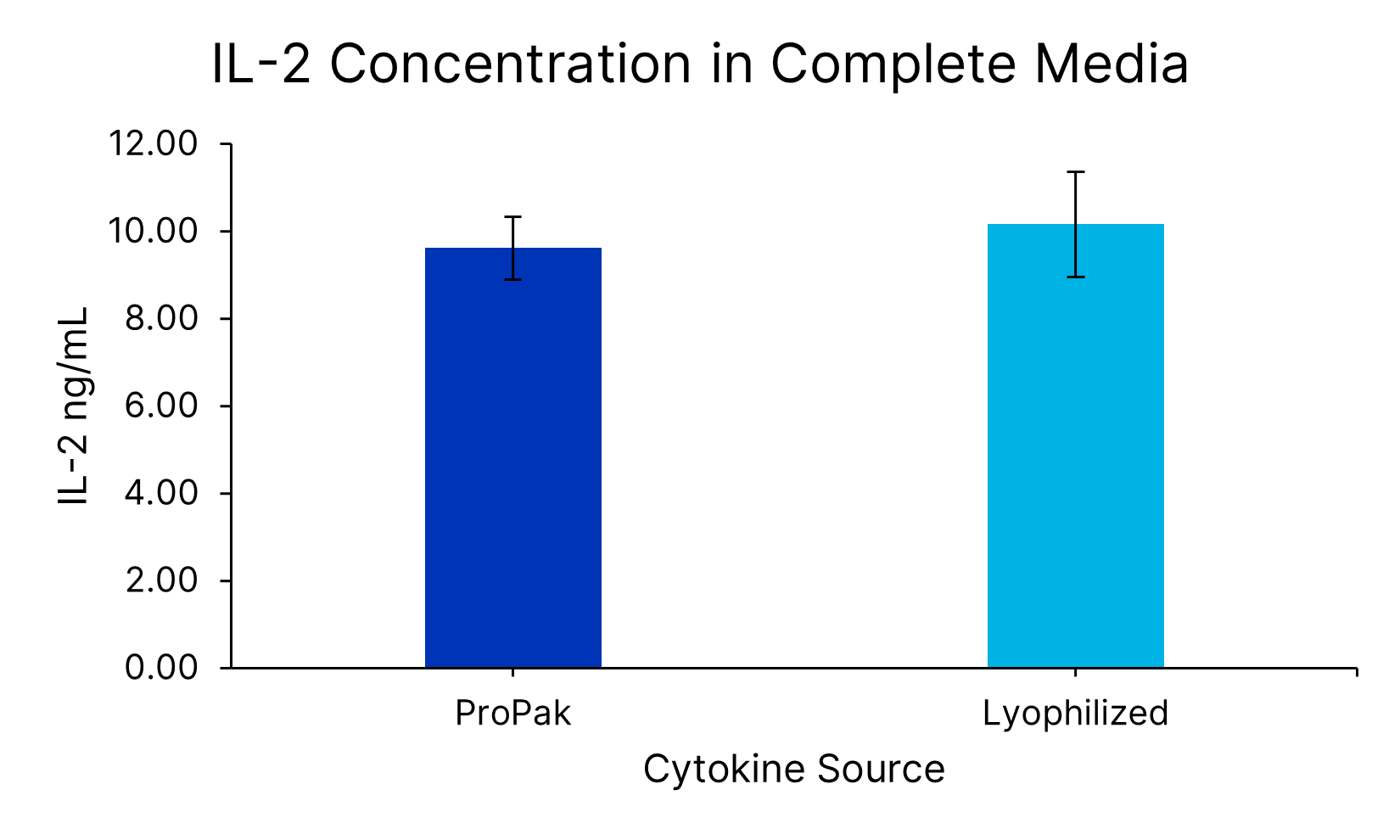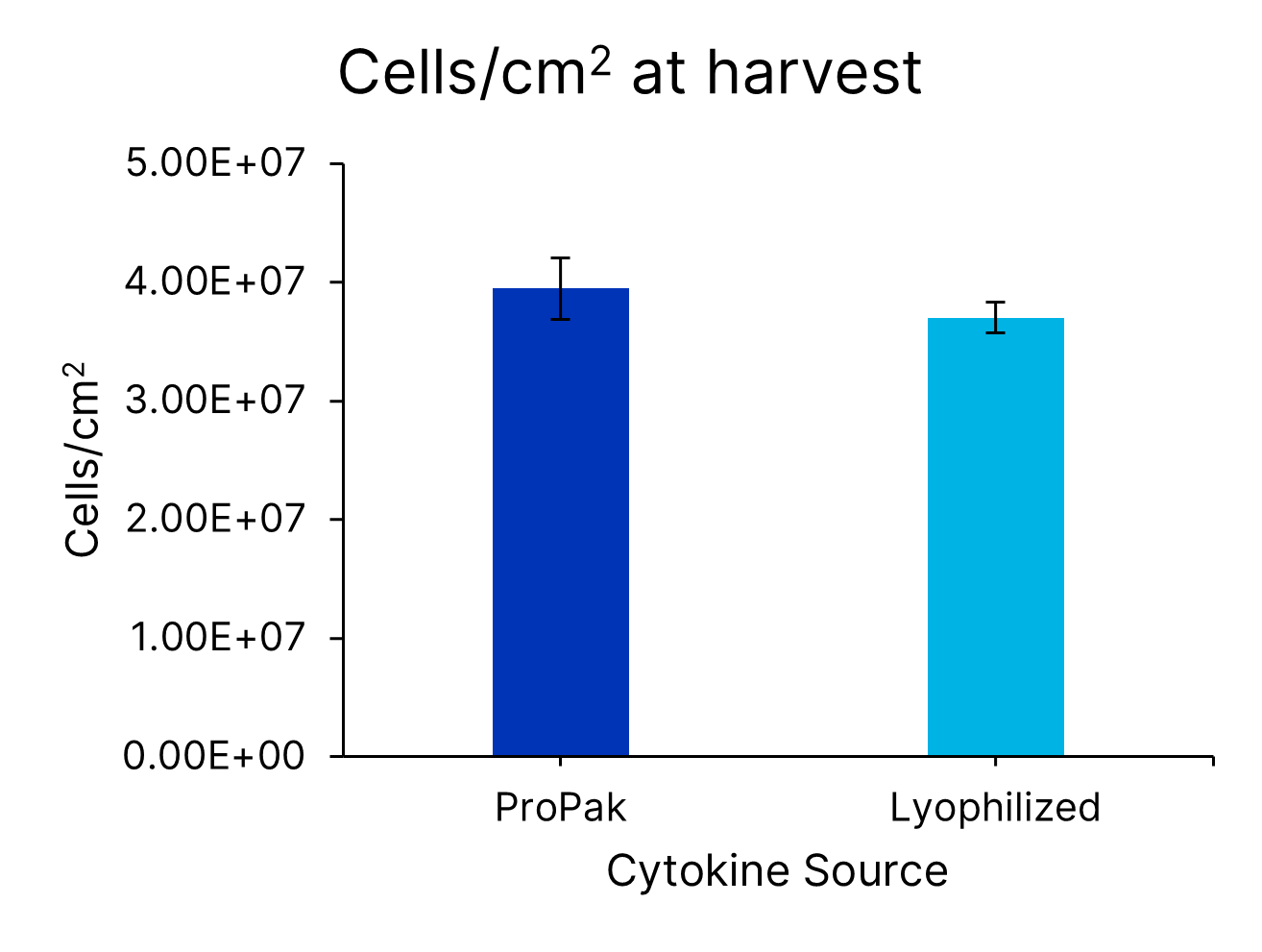ProPak™ Recombinant Human IL-2 GMP Protein GMP
R&D Systems, part of Bio-Techne | Catalog # PPK-002-GMP

Key Product Details
- Closed-System Process In order to de-risk your therapy as much as possible, ProPak GMP cytokines are designed to integrate seamlessly into a closed-system process. The risk of contamination is significantly lowered as a result. Learn more about closed process reagents.
- Weldable Tubing Each ProPak bag is outfitted with weldable tubing, so you can connect it directly to your media, then add both to your bioreactor of choice. Tubing is compatible with any bagged media for use in a 1 L bioreactor, such as the G-Rex.
- Ready-to-Use Format Supplied in a 3mL frozen liquid format. Extensive quality testing guarantees that the liquid formulation is just as potent and stable as lyophilized equivalents, allowing you to safely take away the need for reconstitution steps.
- Reduced Manual Touchpoints By removing both reconstituting and aliquoting steps, there is less hands-on technician time. This drastically reduces risks of error while streamlining your journey to the clinic.
Product Specifications
Source
E. coli-derived human IL-2 protein
Ala21-Thr153 (Cys145Ser), with and without an N-terminal Met
Produced using non-animal reagents in an animal-free laboratory.Manufactured and tested under cGMP guidelines.
Ala21-Thr153 (Cys145Ser), with and without an N-terminal Met
Produced using non-animal reagents in an animal-free laboratory.Manufactured and tested under cGMP guidelines.
Purity
>97%, by SDS-PAGE with quantitative densitometry by Coomassie® Blue Staining. The molecular weight by mass spectrometry is 15521 ± 5 Da, and a second 15390 ± 5 Da product may be present.
Endotoxin Level
<5.0 EU/mL by the LAL method.
N-terminal Sequence Analysis
Met-Ala21-Pro-Thr-Ser-Ser-Ser-Thr-Lys-Lys & Ala21-Pro-Thr-Ser-Ser-Ser-Thr-Lys-Lys-Thr
Predicted Molecular Mass
15.5 kDa
SDS-PAGE
13 kDa, under reducing conditions.
Activity
Measured in a cell proliferation assay using CTLL-2 mouse cytotoxic T cells. Gearing, A.J.H. and C.B. Bird (1987) in Lymphokines and Interferons, A Practical Approach. Clemens, M.J. et al. (eds): IRL Press. 295.
The ED50 for this effect is 0.0300-0.250 ng/mL.
The specific activity of recombinant human IL-2 is >5.00 x 106 IU/mg, which is calibrated against an internal reference standard value assigned against the human IL-2 WHO International Standard (NIBSC code: 86/500).
Host Cell Protein
<0.500 ng per μg of protein when tested by ELISA.
Mycoplasma
Negative for Mycoplasma.
Host Cell DNA
<0.00150 ng per µg of protein when tested by PCR.
Scientific Data Images for ProPak™ Recombinant Human IL-2 GMP Protein
Lot to Lot Consistency
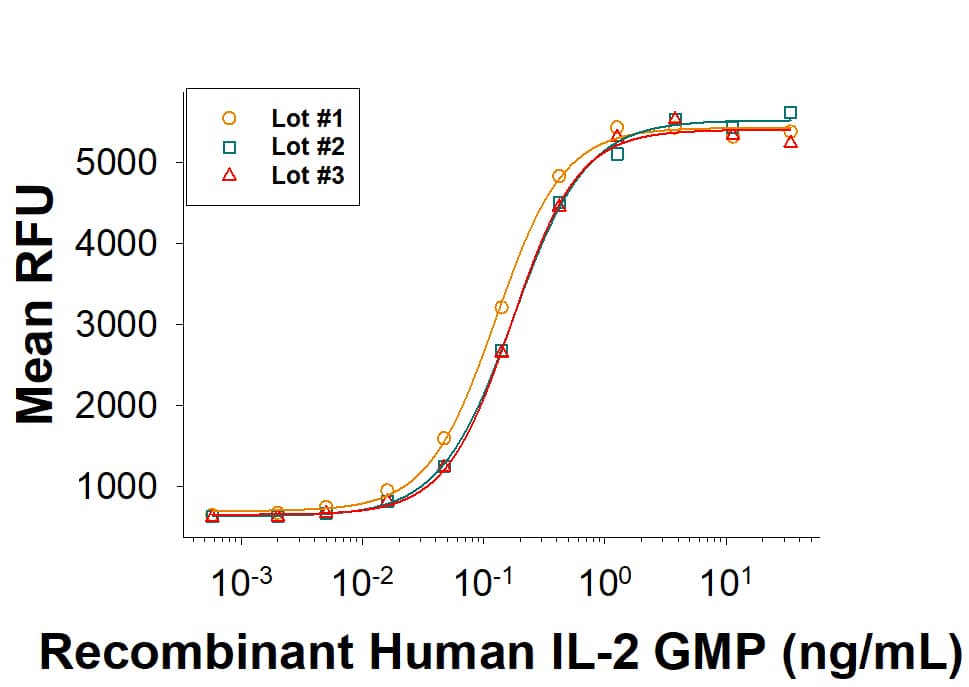
ProPak™ GMP-grade Recombinant Human IL-2 (PPK-002-GMP) stimulates proliferation of CTLL‑2 mouse cytotoxic T cells. The ED50 for this effect is 0.0300-0.250 ng/mL. Three independent lots were tested for activity and plotted on the same graph to show lot-to-lot consistency of ProPak™ GMP IL-2.ProPak™ cytokines are successfully recovered into 1L media bags.
ProPak™ IL-2 (PPK-002-GMP-010) was added to Xeno-free Human T cell media in a bag. In parallel, lyophilized IL-2 (BT-002-GMP) was added to Xeno-free Human T cell media in a bottle (CCM038-GMP-1L). After cytokine addition, the concentration of IL-2 (SPCKB-PS-000295) was determined by the Ella automated immunoassay system. Data is the average of 4 independent experiments with error bars ±SD.ProPak™ cytokines support T cell growth
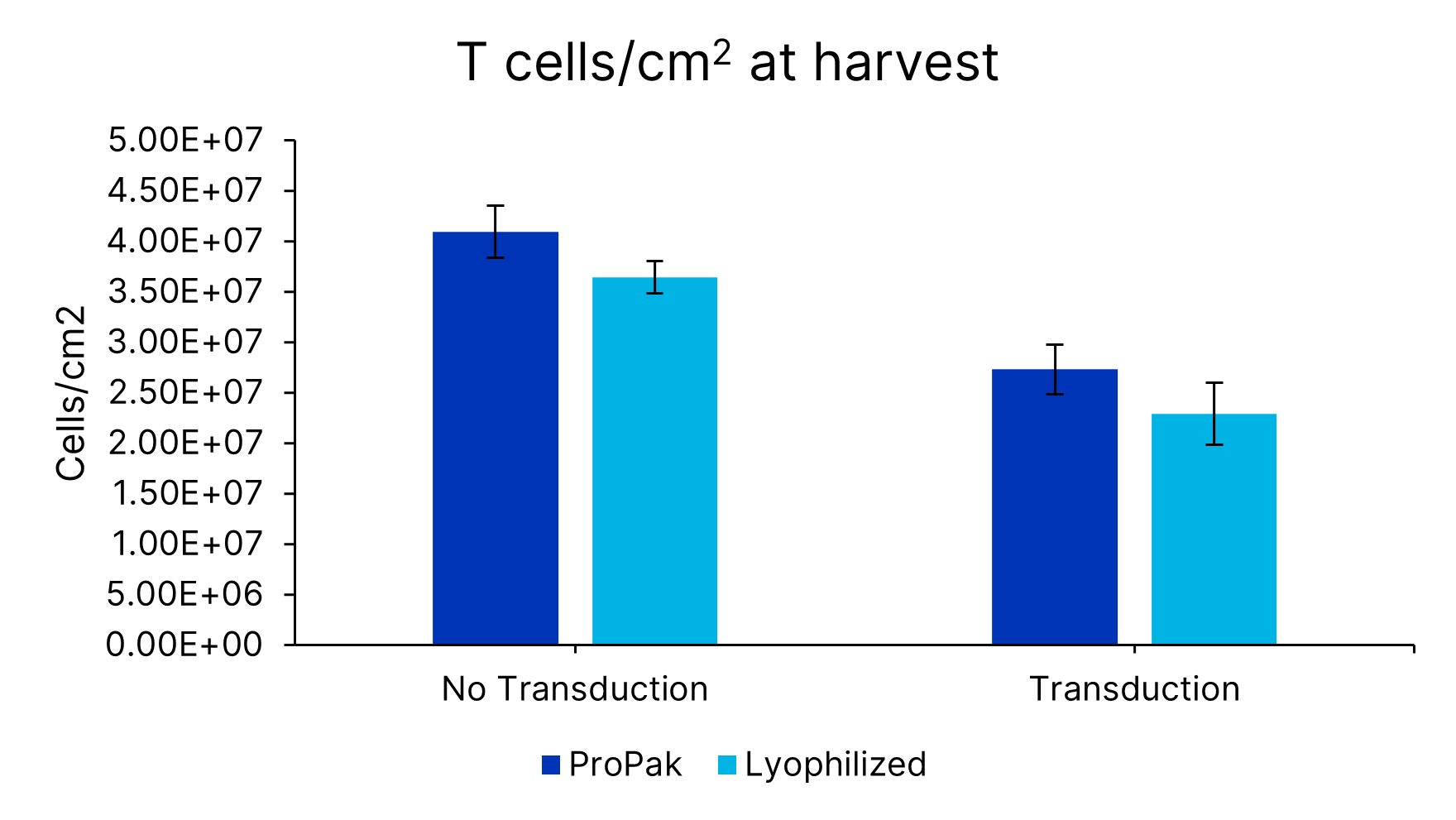
ProPak IL-2 (PPK-002-GMP-010) was added to Xeno-free Human T cell media with 5% human AB serum. In parallel, lyophilized IL-2 (BT-002-GMP) was added to Xeno-free Human T cell media in a bottle (CCM038-GMP-1L). T cells from 3 donors were activated and expanded in G-Rex bioreactors for 7 days and assessed for cells/cm2 and cell viability. Data is the average of 4 independent experiments with 3 donors per experiment, error bars ±SD.Formulation, Preparation and Storage
PPK-002-GMP
| Formulation | Supplied as a 0.2 μm filtered solution in PBS, recombinant HSA, and Trehalose. |
| Reconstitution | |
| Shipping | This product is shipped on dry ice. Upon receipt, store immediately at the temperature recommended below. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles. A minimum of 6 months when stored between -14 °C and -40 °C. Can be stored up to 2 weeks at 2-8 °C. Refer to lot specific COA for the Use by Date. |
Background: IL-2
References
- Ma, A. et al. (2006) Annu. Rev. Immunol. 24:657.
- Gaffen, S.L. and K.D. Liu (2004) Cytokine 28:109.
- Taniguchi, T. et al. (1983) Nature 302:305.
- Mosmann, T.R. et al. (1987) J. Immunol. 138:1813.
- Liparoto, S.F. et al. (2002) Biochemistry 41:2543.
- Wang, X. et al. (2005) Science 310:1159.
- Bodnar, A. et al. (2008) Immunol. Lett. 116:117.
- Jaleco, S. et al. (2003) J. Immunol. 171:61.
- Malek, T.R. (2003) J. Leukoc. Biol. 74:961.
- Laurence, A. et al. (2007) Immunity 26:371.
- Kryczek, I. et al. (2007) J. Immunol. 178:6730.
- Afzali, B. et al. (2007) Clin. Exp. Immunol. 148:32.
- Fehervari, Z. et al. (2006) Trends Immunol. 27:109.
- Xue, D. et al. (2021) Antib Ther. 4:123.
- Wolfarth, A.A. et al. (2022) Immune Netw. 22:e5.
- Koehl, U. et al. (2015) Oncoimmunology. 5:e1115178.
- Marsman, C. et al. (2022) Front. Immunol. 13:815449.
- Chamucero-Millares, J.A.et al. (2021) Cell Immunol. 360:104257
Long Name
Interleukin 2
Alternate Names
Aldesleukin, IL2, Proleukin, TCGF
Entrez Gene IDs
Gene Symbol
IL2
UniProt
Additional IL-2 Products
Product Documents for ProPak™ Recombinant Human IL-2 GMP Protein
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Designed, manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented and controlled manufacturing process
- Control of documentation and process changes by QA
- Personnel training programs
- Raw material inspection and vendor qualification/monitoring program
- Validated equipment, processes and test methods
- Equipment calibration and maintenance schedules using a Regulatory Asset Manager
- Facility/Utilities maintenance, contamination controls, safety and pest control programs
- Material review process for variances
- Robust product stability program following relevant ICH guidelines
- N-terminal amino acid analysis
- SDS-PAGE purity analysis
- Molecular weight analysis via mass spectrometry
- Endotoxin assessment per USP <85> and Ph. Eur. 2.6.14 guidelines
- Bioassay analysis
- Microbial testing per USP <71> and Ph. Eur. 2.6.1 guidelines
- Host cell protein assessment
- Host cell DNA assessment
- Mycoplasma assessment
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis and St. Paul, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Animal-Free Manufacturing Conditions
Our dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers.
Product Specific Notices for ProPak™ Recombinant Human IL-2 GMP Protein
Full terms and conditions of sale can be found online in the Protein Sciences Segment T&Cs at: Terms & Conditions.
For preclinical, or clinical ex vivo use
Loading...
Loading...
Loading...